
Is $HN{O_3}$ a coordinate bond?
Answer
471.3k+ views
Hint: The nitrogen atom is bonded to three oxygen atoms; it's bonded to at least one via one chemical bond, another via a double chemical bond, and another by a dative (coordinate) chemical bond. there's one coordinate chemical bond in aqua fortis.
Complete answer:
Ozone which isn’t attached to the hydrogen and therefore the one which isn't a double bone with nitrogen. This leaves one oxygen which has three non-bonding electron pairs of electrons. This oxygen RECEIVES the non-bonding pair that also remains on the NITROGEN atom after it's formed a covalent bond with one among the oxygens and one bond with the opposite oxygen which successively is bonded to the hydrogen.
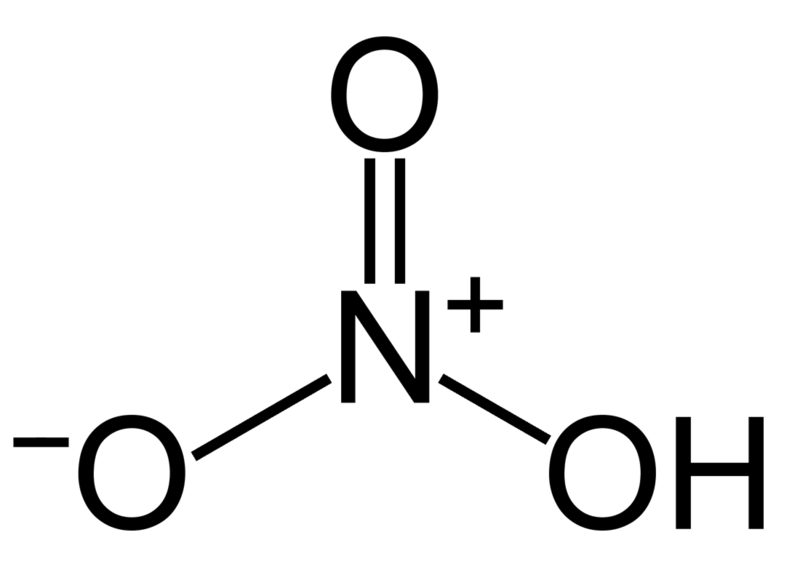
The $HN{O_3}$ Lewis structure is best thought of because the $N{O_3}$ with $H$ attached to at least one of the oxygen atoms. this is often a pattern seen with many acids. For the $HN{O_3}$ Lewis structure, calculate the entire number of valence electrons for the $HN{O_3}$ molecule. After determining what percentage valence electrons there are in $HN{O_3}$, place them around the central atom to finish the octets. make certain to use the number of obtainable valence electrons you found earlier. The $HN{O_3}$ Lewis structure has 24 valence electrons.
Note:
A chemical bond is made by two atoms sharing a pair of electrons. The atoms are held
together because the electron pair is attracted by both of the nuclei. within the formation of an easy chemical bond, each atom supplies one electron to the bond - but that doesn't need to be the case.
Complete answer:
Ozone which isn’t attached to the hydrogen and therefore the one which isn't a double bone with nitrogen. This leaves one oxygen which has three non-bonding electron pairs of electrons. This oxygen RECEIVES the non-bonding pair that also remains on the NITROGEN atom after it's formed a covalent bond with one among the oxygens and one bond with the opposite oxygen which successively is bonded to the hydrogen.

The $HN{O_3}$ Lewis structure is best thought of because the $N{O_3}$ with $H$ attached to at least one of the oxygen atoms. this is often a pattern seen with many acids. For the $HN{O_3}$ Lewis structure, calculate the entire number of valence electrons for the $HN{O_3}$ molecule. After determining what percentage valence electrons there are in $HN{O_3}$, place them around the central atom to finish the octets. make certain to use the number of obtainable valence electrons you found earlier. The $HN{O_3}$ Lewis structure has 24 valence electrons.
Note:
A chemical bond is made by two atoms sharing a pair of electrons. The atoms are held
together because the electron pair is attracted by both of the nuclei. within the formation of an easy chemical bond, each atom supplies one electron to the bond - but that doesn't need to be the case.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




