
Is methanol a polar molecule?
Answer
492.6k+ views
Hint: Methanol, also known as \[C{H_3}OH\] , is a flammable, colorless, and volatile liquid with a pronounced alcoholic odor. The molecular structure, bond angle, and polarity of the molecule can all be determined by analyzing its Methanol. The polarity of \[C{H_3}OH\] is an important property since it aids in determining the compound's other properties, such as its solubility, electric charges, and so on. Let's look at the bond angles and arrangement of the atoms in the \[C{H_3}OH\] molecule to better comprehend Methanol's polarity.
Complete answer:
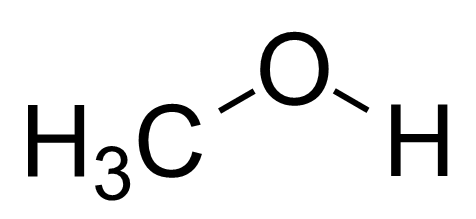
The polarity of any given chemical is determined by the complex's net dipole moment. The electric charges on the atoms in the molecule can be used to calculate the net dipole moment. In Methanol, both the Carbon and Oxygen atoms (which act as geometric centers for the chemical) are electronegative. However, oxygen in the molecule is more electronegative than carbon or hydrogen.
Because the atoms in the methanol molecule have different electric charges, \[C{H_3}OH\] cannot be non-polar. Because of its two lone pairs of electrons, oxygen has a higher electron density. \[C{H_3}OH\] becomes polar as a result of the net dipole pointing towards the Oxygen atom.
Apart from the electric charges, the asymmetry of the CH3OH molecule eliminates the possibility of non-polarity. The dipole-dipole moment cancels out in a nonpolar molecule, giving it a symmetrical shape. However, because Methanol has a bend in its shape, it forms an asymmetric structure, with the negative end of the net electric dipole moment pointing towards the Oxygen atom. Hence, methanol is a polar molecule.
Note:
Physical property of Methanol:
Methanol (\[C{H_3}OH\] ) has a boiling point of \[{64.7^ \circ }C\]
Methanol has a melting point of \[ - {97.6^ \circ }C\]
Methanol has a molecular weight of \[32.04\dfrac{g}{{mol}}\] .
It's a polar solvent that's also known as wood alcohol due to the fact that it was originally made by distilling wood.
When compared to ethanol, the fragrance of this molecule is sweeter.
Complete answer:

The polarity of any given chemical is determined by the complex's net dipole moment. The electric charges on the atoms in the molecule can be used to calculate the net dipole moment. In Methanol, both the Carbon and Oxygen atoms (which act as geometric centers for the chemical) are electronegative. However, oxygen in the molecule is more electronegative than carbon or hydrogen.
Because the atoms in the methanol molecule have different electric charges, \[C{H_3}OH\] cannot be non-polar. Because of its two lone pairs of electrons, oxygen has a higher electron density. \[C{H_3}OH\] becomes polar as a result of the net dipole pointing towards the Oxygen atom.
Apart from the electric charges, the asymmetry of the CH3OH molecule eliminates the possibility of non-polarity. The dipole-dipole moment cancels out in a nonpolar molecule, giving it a symmetrical shape. However, because Methanol has a bend in its shape, it forms an asymmetric structure, with the negative end of the net electric dipole moment pointing towards the Oxygen atom. Hence, methanol is a polar molecule.
Note:
Physical property of Methanol:
Methanol (\[C{H_3}OH\] ) has a boiling point of \[{64.7^ \circ }C\]
Methanol has a melting point of \[ - {97.6^ \circ }C\]
Methanol has a molecular weight of \[32.04\dfrac{g}{{mol}}\] .
It's a polar solvent that's also known as wood alcohol due to the fact that it was originally made by distilling wood.
When compared to ethanol, the fragrance of this molecule is sweeter.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




