
Is $Mg{H_2}$ ionic?
Answer
504k+ views
Hint: To gain stability, to complete the octet rule all the atoms share electrons to gain the noble gas configuration. Also called the eighteen electrons to rule. The sharing of this electron is called a bond. However, it is not mandatory that both the atoms donate equal numbers of electrons like in the case of coordination bond.
Complete answer:
After the sharing of electrons, the constituents are held together by a force of attraction, these attractive forces are termed as chemical bonds. The successful explanation of the chemical bond was for the first time explained by Kossel and Lewis. Then later on both of them gave their combined theory of octet rule. However, this law found many limitations.
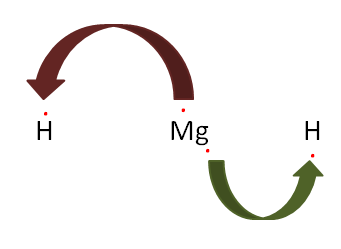
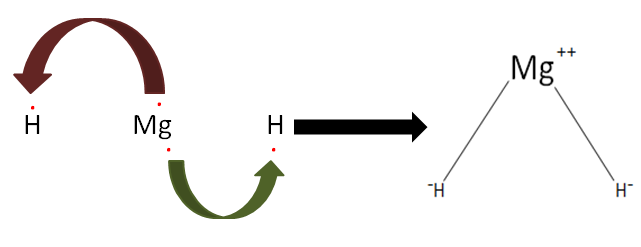
When in a bond there is a complete transfer of electrons from one atom to another, then it is called an Ionic bond. But if the complete transfer of electrons is not observed and there is sharing of electrons then they are said to be covalent bonds. But if in this sharing all the electrons are contributed by one atom only and another atom just accepts it only is said to be a covalent bond.
Note:
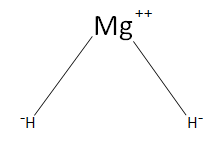
$Mg{H_2}$ in ionic in nature with two ionic bonds and zero covalent bonds. The formation of an ionic bond depends on the ionization enthalpy, electron gain enthalpy, lattice enthalpy respectively. Though it is complicated to make an ion like this only, we need to provide very high energy to the atom so that it is converted to an ion. However, ions play a vital role in the growth and development of an organism, sodium-potassium pump is an example.
Complete answer:
After the sharing of electrons, the constituents are held together by a force of attraction, these attractive forces are termed as chemical bonds. The successful explanation of the chemical bond was for the first time explained by Kossel and Lewis. Then later on both of them gave their combined theory of octet rule. However, this law found many limitations.
When in a bond there is a complete transfer of electrons from one atom to another, then it is called an Ionic bond. But if the complete transfer of electrons is not observed and there is sharing of electrons then they are said to be covalent bonds. But if in this sharing all the electrons are contributed by one atom only and another atom just accepts it only is said to be a covalent bond.



Note:
$Mg{H_2}$ in ionic in nature with two ionic bonds and zero covalent bonds. The formation of an ionic bond depends on the ionization enthalpy, electron gain enthalpy, lattice enthalpy respectively. Though it is complicated to make an ion like this only, we need to provide very high energy to the atom so that it is converted to an ion. However, ions play a vital role in the growth and development of an organism, sodium-potassium pump is an example.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




