
Is the \[O - H\] bond in methanol polar or nonpolar?
Answer
494.4k+ views
Hint: Compounds formed by some type of attraction between atoms, ions or molecules, this attraction is termed as chemical bond. These bonds have fixed shape and are directional. These are of two types which are polar and nonpolar bonds.
Complete answer:
For this question, we have to following steps:
Step-1: Firstly we have to sketch the Lewis structure of the given compound, i.e. $C{H_3}OH$
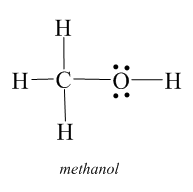
Step-2: To identify the polarity of each bond, i.e. whether the bond is polar or nonpolar for that we will check the difference in electronegativity between the bonds. If the difference is more than $0.4$ , then the bond would be considered as polar in nature but if the difference is less than $0.4$, then it will be nonpolar in nature.
In the given compound, i.e. $C{H_3}OH$, to find the difference in the electronegativity between the \[O - H\]bond we will first find the value of electronegativity of $O$ and after that of $H$ . So the electronegativity of $O$ is $3.44$ and the electronegativity of $H$ is $2.2$ . So the difference in electronegativity is $1.24$. Therefore, the bond \[O - H\] is polar in nature.
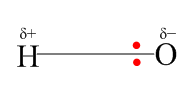
Moreover we know that the oxygen atom is more electronegative than the hydrogen atom so electrons are more close to the oxygen atom, this results in a dipole. Hence, resulting in the partial negative charge on the oxygen atom and partial positive on the hydrogen atom. Thus, forming the \[O - H\] bond polar in nature.
The structural representation of \[O - H\] bond is as follows:
Note:
It is important to remember that the Lewis structure of the compound may give the wrong impression of the molecule’s geometry. So, the Lewis structures are mostly used for finding the polarity of the bond whereas geometry can predict the polarity of the overall molecule.
Complete answer:
For this question, we have to following steps:
Step-1: Firstly we have to sketch the Lewis structure of the given compound, i.e. $C{H_3}OH$

Step-2: To identify the polarity of each bond, i.e. whether the bond is polar or nonpolar for that we will check the difference in electronegativity between the bonds. If the difference is more than $0.4$ , then the bond would be considered as polar in nature but if the difference is less than $0.4$, then it will be nonpolar in nature.
In the given compound, i.e. $C{H_3}OH$, to find the difference in the electronegativity between the \[O - H\]bond we will first find the value of electronegativity of $O$ and after that of $H$ . So the electronegativity of $O$ is $3.44$ and the electronegativity of $H$ is $2.2$ . So the difference in electronegativity is $1.24$. Therefore, the bond \[O - H\] is polar in nature.
Moreover we know that the oxygen atom is more electronegative than the hydrogen atom so electrons are more close to the oxygen atom, this results in a dipole. Hence, resulting in the partial negative charge on the oxygen atom and partial positive on the hydrogen atom. Thus, forming the \[O - H\] bond polar in nature.
The structural representation of \[O - H\] bond is as follows:

Note:
It is important to remember that the Lewis structure of the compound may give the wrong impression of the molecule’s geometry. So, the Lewis structures are mostly used for finding the polarity of the bond whereas geometry can predict the polarity of the overall molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




