
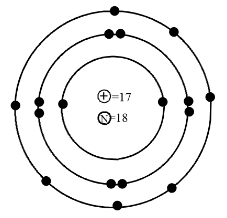
Is this Bohr’s atomic structure of Cl right?

Answer
495.3k+ views
Hint: Neil Bohr gave a planetary model to explain the rotation of electrons around the nucleus. This model explains how the electrons rotate in a circular orbit. In the above question Bohr’s model form chlorine atom is given to see that all the electrons are paired in the diagram.
Complete answer:
Neil Bohr gave a model of all the atoms known as Bohr’s model of an atom. It is a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus similar to the planets orbiting the sun. This model explains that the electrons travel in definite circular orbits around the nucleus. The model of the atom is based on quantum mechanics.
In the question we are given Bohr’s model of Chlorine, but this model is not correct for the chlorine atoms. In this diagram the number of shells, electrons, protons and neutrons are correct but the default is in the placement of electrons. In the diagram the first shell has two single electrons but they should be together in pairs. Then in the third shell there are seven electrons. All these electrons are placed single but among them six electrons should be together in pairs, forming the three sets of double electrons and there should be only one single electron.
Therefore the correct diagram for the Bohr’s model of the chlorine atom is:
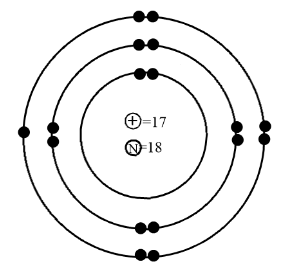
We can see in the above diagram that all the electrons are in pairs in all the three shells and there is only one single electron. So this model is the correct model of the chlorine atoms.
Note:
Bohr’s model successfully explains the rotation of electrons around the nucleus but it has certain limitations also. It does not explain the spectra of atoms having more than one electron. It did not make correct predictions about the large-sized atoms and did not provide sufficient information for smaller atoms.
Complete answer:
Neil Bohr gave a model of all the atoms known as Bohr’s model of an atom. It is a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus similar to the planets orbiting the sun. This model explains that the electrons travel in definite circular orbits around the nucleus. The model of the atom is based on quantum mechanics.
In the question we are given Bohr’s model of Chlorine, but this model is not correct for the chlorine atoms. In this diagram the number of shells, electrons, protons and neutrons are correct but the default is in the placement of electrons. In the diagram the first shell has two single electrons but they should be together in pairs. Then in the third shell there are seven electrons. All these electrons are placed single but among them six electrons should be together in pairs, forming the three sets of double electrons and there should be only one single electron.
Therefore the correct diagram for the Bohr’s model of the chlorine atom is:

We can see in the above diagram that all the electrons are in pairs in all the three shells and there is only one single electron. So this model is the correct model of the chlorine atoms.
Note:
Bohr’s model successfully explains the rotation of electrons around the nucleus but it has certain limitations also. It does not explain the spectra of atoms having more than one electron. It did not make correct predictions about the large-sized atoms and did not provide sufficient information for smaller atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




