
Isobutyl group is a ……….. group.
A. primary
B. secondary
C. tertiary
D. functional
Answer
558.6k+ views
Hint: Organic compounds are named based on a system known as International Union of Pure and Applied Chemistry which is abbreviated as IUPAC. The major part of the name of the compound is the word root or parent name.
Complete step by step answer:
Usually, the IUPAC names of the organic compounds consist of four parts-prefix, word root, primary suffix and secondary suffix. Primary suffix signifies the nature of the carbon atom chain. When there is a single bond between the carbon atoms, the primary suffix will be ‘’ane’’. When there is a double bond between them, then it will be ‘’ene’’. If it is a triple bond, it will be ‘’yne’’. Secondary suffix indicates the functional group of the compound.
When the prefix is considered, it is of two types-primary prefix and secondary prefix. It may be ‘’cyclo’’ or any others depending upon the functional groups.
In the given question, the root name is ‘’but’’. So the total number of carbon atoms is four. Butyl is a group which is made from different isomers of butane.
Primary group is the group in which the carbon atom is attached to only one carbon atom. In the secondary group, the carbon atom is attached to two carbon atoms. While in tertiary and quaternary groups, the carbon atom is attached to three, four carbon atoms respectively.
The structure of isobutyl group is given below:
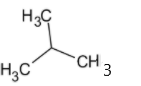
Thus the isobutyl group is a primary group.
So, the correct answer is Option A.
Note: The table given below represents the number of carbon atoms and its corresponding root name:
Complete step by step answer:
Usually, the IUPAC names of the organic compounds consist of four parts-prefix, word root, primary suffix and secondary suffix. Primary suffix signifies the nature of the carbon atom chain. When there is a single bond between the carbon atoms, the primary suffix will be ‘’ane’’. When there is a double bond between them, then it will be ‘’ene’’. If it is a triple bond, it will be ‘’yne’’. Secondary suffix indicates the functional group of the compound.
When the prefix is considered, it is of two types-primary prefix and secondary prefix. It may be ‘’cyclo’’ or any others depending upon the functional groups.
In the given question, the root name is ‘’but’’. So the total number of carbon atoms is four. Butyl is a group which is made from different isomers of butane.
Primary group is the group in which the carbon atom is attached to only one carbon atom. In the secondary group, the carbon atom is attached to two carbon atoms. While in tertiary and quaternary groups, the carbon atom is attached to three, four carbon atoms respectively.
The structure of isobutyl group is given below:

Thus the isobutyl group is a primary group.
So, the correct answer is Option A.
Note: The table given below represents the number of carbon atoms and its corresponding root name:
| Number of carbon atoms | Root name |
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
| 6 | Hex |
| 7 | Hept |
| 8 | Oct |
| 9 | Non |
| 10 | Dec |
| 11 | Undec |
| 12 | Dodec |
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




