
How many isomers can you draw for pentane?
a. 3
b. 2
c. 4
d. 5
Answer
585.6k+ views
Hint: As the name implies pent which means five hence, pentane has five carbon atoms. Structural isomers have the same molecular formula but have a different arrangement of atoms so we can use this idea to arrange the carbon atom of pentane to form different structural isomers.
Complete step by step answer: Let's learn about isomers in more detail. so, isomers are the molecules or compounds that have the same formula but have different structures through atoms connected in a different order, that is their bonding structures are different here the bonding structure refers to the way that atoms are bonded in the molecule.
Pentane is an organic compound that comes under the category of alkanes. Alkanes are those organic compounds which do not have a double bond or a triple bond and thus, these alkanes are called saturated compounds.
The molecular formula of pentane is ${{C}_{5}}{{H}_{12}}$. There are five carbons and there are no other functional groups in pentane. We can arrange these five carbon atoms differently in three ways to form 3 different structural isomers of pentane.
Let’s discuss this arrangement one by one.
- In the first arrangement, the carbon should be linked with one another to form a straight chain in this for isomer is called n-pentane and its formula is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. The structure of this isomer is given below.
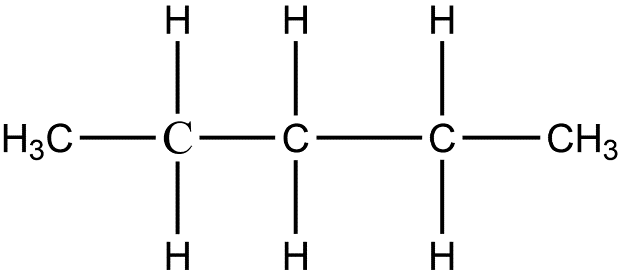
- Now in the second arrangement one carbon atom is branched with the second carbon atom of the straight-chain and the rest four carbon atoms in pentane are in a straight line. This isomer is known as iso-pentane and its formula is $C{{H}_{3}}CH(C{{H}_{3}})C{{H}_{2}}C{{H}_{3}}$. Here is the structure of iso-pentane.
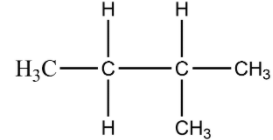
- In the last arrangement, three of the carbon atoms are in the straight-chain and two carbon atoms are branched with the central carbon atom of the straight chain. This isomer is known as neo-pentane. And its molecular formula is $C{{H}_{3}}C{{(C{{H}_{3}})}_{2}}C{{H}_{3}}$. The structure of neopentane is given below:
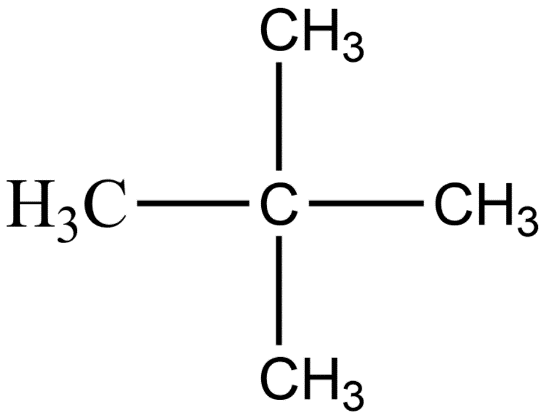
Therefore, as we can see that there are 3 isomers of pentane hence option a) is the correct one.
Note: For branched carbon atoms in a straight chain, the carbon atoms are numbered from the last carbon which is closest to the branched carbon.
While drawing isomers make sure that every isomer is distinct. Avoid confusing between these two structures and also do not count them twice while finding out the number of structural isomers. Let's take the isopentane as an example.
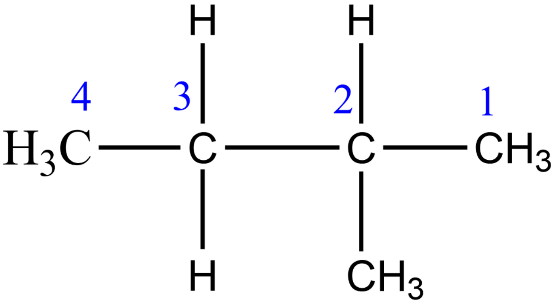
So, it is the correct way of numbering.
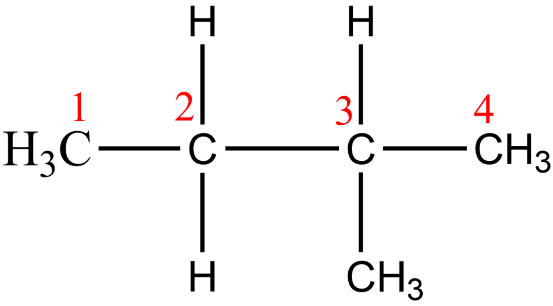
And, this is the wrong way of numbering.
Complete step by step answer: Let's learn about isomers in more detail. so, isomers are the molecules or compounds that have the same formula but have different structures through atoms connected in a different order, that is their bonding structures are different here the bonding structure refers to the way that atoms are bonded in the molecule.
Pentane is an organic compound that comes under the category of alkanes. Alkanes are those organic compounds which do not have a double bond or a triple bond and thus, these alkanes are called saturated compounds.
The molecular formula of pentane is ${{C}_{5}}{{H}_{12}}$. There are five carbons and there are no other functional groups in pentane. We can arrange these five carbon atoms differently in three ways to form 3 different structural isomers of pentane.
Let’s discuss this arrangement one by one.
- In the first arrangement, the carbon should be linked with one another to form a straight chain in this for isomer is called n-pentane and its formula is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. The structure of this isomer is given below.

- Now in the second arrangement one carbon atom is branched with the second carbon atom of the straight-chain and the rest four carbon atoms in pentane are in a straight line. This isomer is known as iso-pentane and its formula is $C{{H}_{3}}CH(C{{H}_{3}})C{{H}_{2}}C{{H}_{3}}$. Here is the structure of iso-pentane.

- In the last arrangement, three of the carbon atoms are in the straight-chain and two carbon atoms are branched with the central carbon atom of the straight chain. This isomer is known as neo-pentane. And its molecular formula is $C{{H}_{3}}C{{(C{{H}_{3}})}_{2}}C{{H}_{3}}$. The structure of neopentane is given below:

Therefore, as we can see that there are 3 isomers of pentane hence option a) is the correct one.
Note: For branched carbon atoms in a straight chain, the carbon atoms are numbered from the last carbon which is closest to the branched carbon.
While drawing isomers make sure that every isomer is distinct. Avoid confusing between these two structures and also do not count them twice while finding out the number of structural isomers. Let's take the isopentane as an example.

So, it is the correct way of numbering.

And, this is the wrong way of numbering.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




