
How many isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas? (Excluding stereoisomer)
Answer
528.7k+ views
Hint: Grignard’s reagents $RMgX$ are very reactive organometallic compounds with carbon-metal bonds. They can react with acidic hydrogen of alcohols to give corresponding hydrocarbons. A general chemical equation for reaction between alcohol and Grignard reagent is
\[{{R}^{\delta -}}-M{{g}^{\delta +}}{{X}^{\delta -}}+R{{O}^{\delta -}}-{{H}^{\delta +}}\to R-H+Mg(OR)X\]
Complete answer:
Let us first find the total number of isomers possible with the chemical formula given, i.e. ${{C}_{4}}{{H}_{10}}O$
General formulas for alcohols and ethers can be given as ${{C}_{n}}{{H}_{n+2}}O$. The chemical formula given to us ${{C}_{4}}{{H}_{10}}O$ fits the general chemical formula for both alcohols and ethers as ${{C}_{4}}{{H}_{2\times 4+2}}O$, therefore, we can say the ${{C}_{4}}{{H}_{10}}O$ is an alcohol or ether.
The possible structures of alcohols with ${{C}_{4}}{{H}_{10}}O$ are shown below:
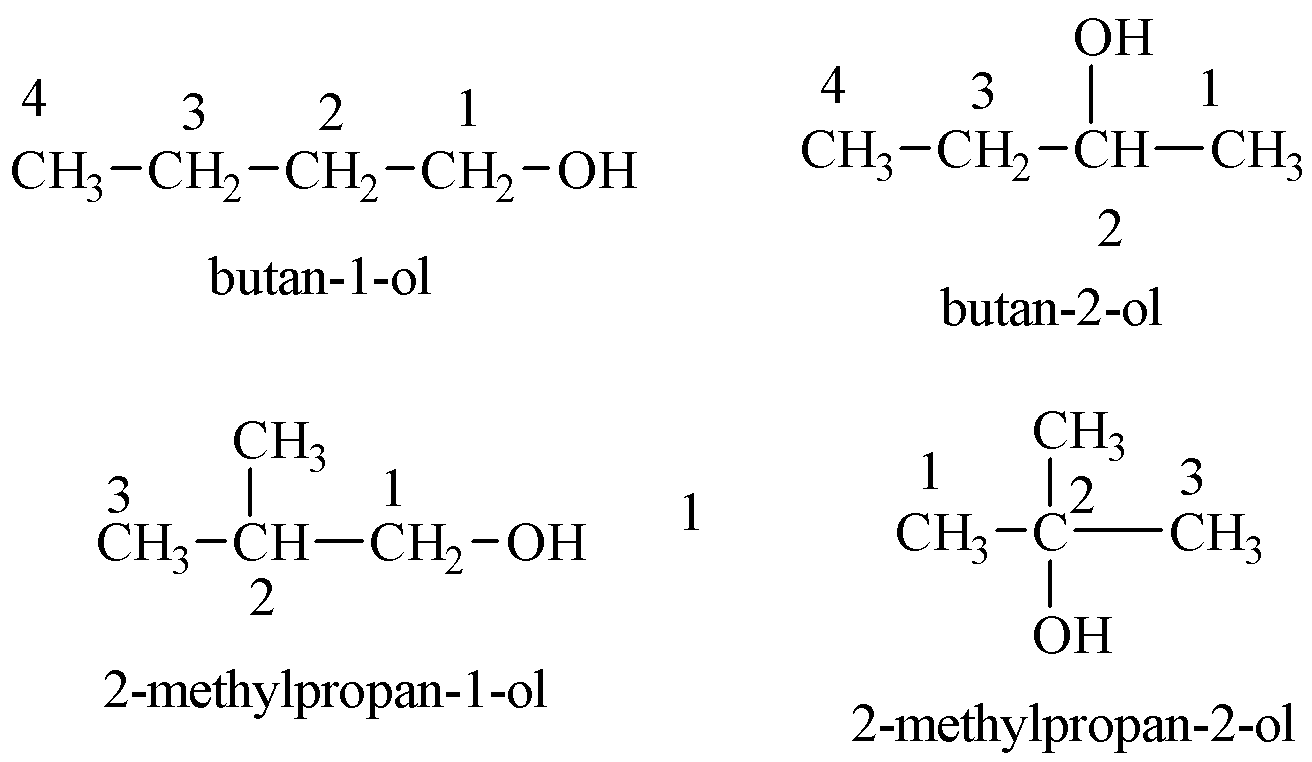
Structures of ethers possible with the chemical formula are given below:
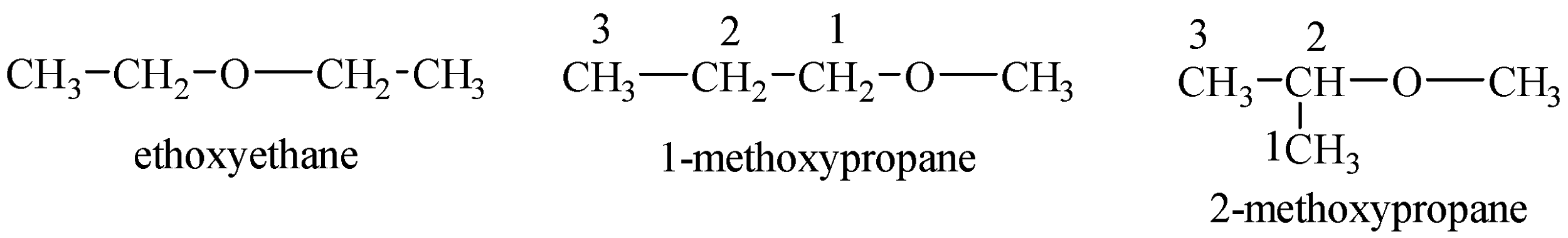
Now, we can see that ethers do not contain any acidic hydrogen and cannot give protons to Grignard’s reagent which is $C{{H}_{3}}MgBr$ in the given question.
Alcohols are acidic in nature due to the polar $O-H$ bond, therefore, can react with Grignard’s reagent $C{{H}_{3}}MgBr$ to give release ($C{{H}_{4}}$) gas. Reactions of all the four alcohols with $C{{H}_{3}}MgBr$ are given below:
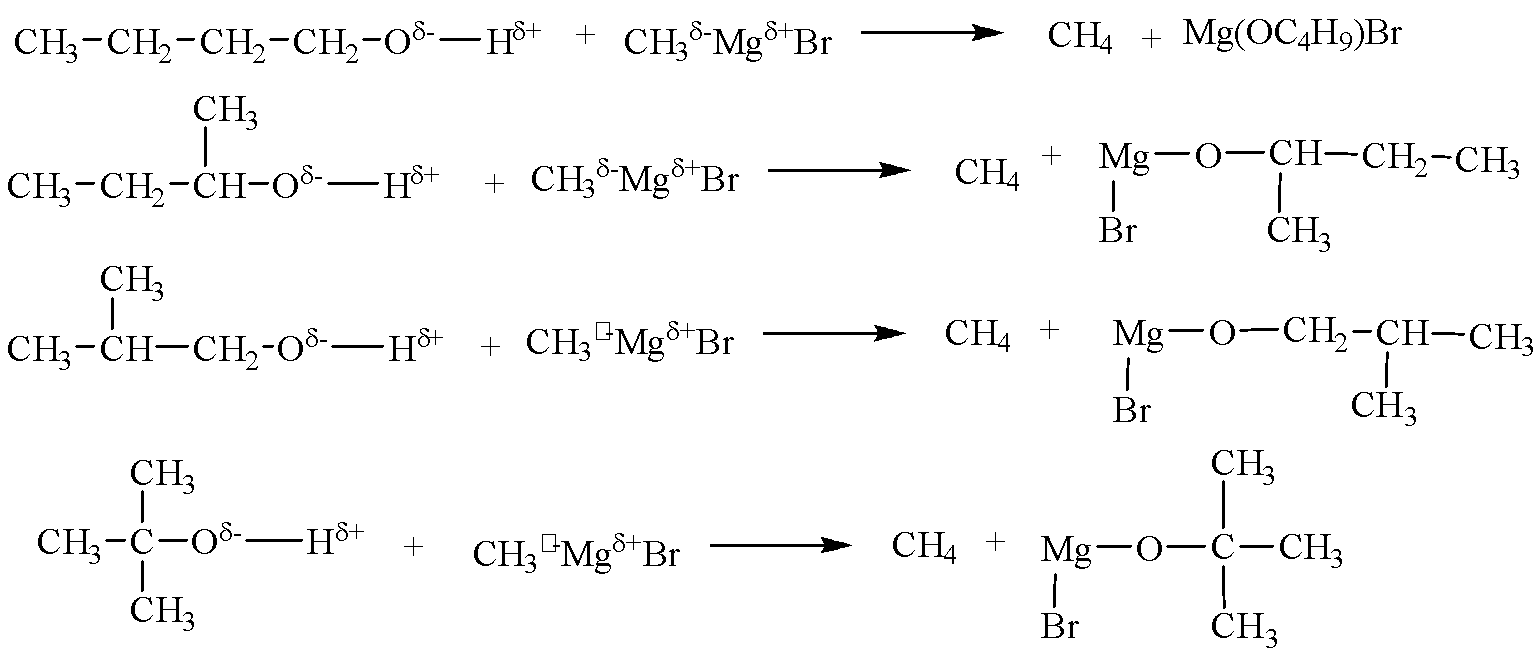
Therefore, four out of the seven isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas.
So, the correct answer is “Option B”.
Additional Information: One stereoisomer of butan-1-ol is possible as it has a chiral carbon. Stereoisomers have the same molecular and structural formula, i.e. have connectivity of atoms but different spatial arrangement of atoms.
Note: In order to react with $C{{H}_{3}}MgBr$, isomers of ${{C}_{4}}{{H}_{10}}O$ must have an acidic hydrogen. Ethers do not contain any acidic hydrogen as the electronegative O is connected to an alkyl chain, hence, they do not react with Grignard’s reagent.
\[{{R}^{\delta -}}-M{{g}^{\delta +}}{{X}^{\delta -}}+R{{O}^{\delta -}}-{{H}^{\delta +}}\to R-H+Mg(OR)X\]
Complete answer:
Let us first find the total number of isomers possible with the chemical formula given, i.e. ${{C}_{4}}{{H}_{10}}O$
General formulas for alcohols and ethers can be given as ${{C}_{n}}{{H}_{n+2}}O$. The chemical formula given to us ${{C}_{4}}{{H}_{10}}O$ fits the general chemical formula for both alcohols and ethers as ${{C}_{4}}{{H}_{2\times 4+2}}O$, therefore, we can say the ${{C}_{4}}{{H}_{10}}O$ is an alcohol or ether.
The possible structures of alcohols with ${{C}_{4}}{{H}_{10}}O$ are shown below:

Structures of ethers possible with the chemical formula are given below:
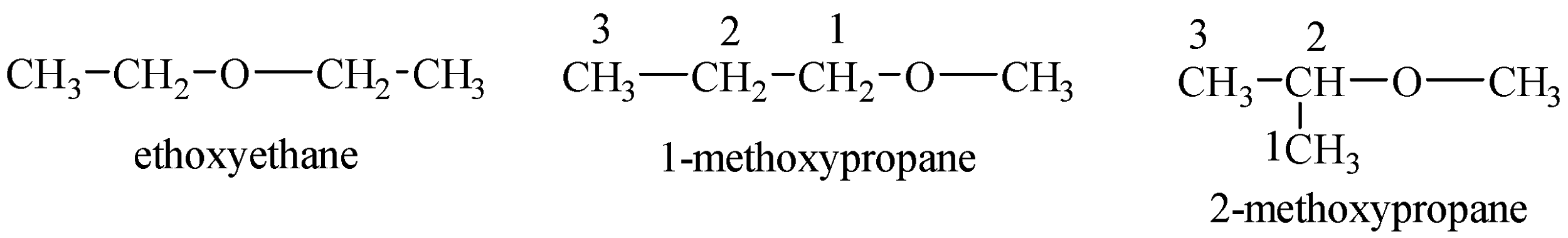
Now, we can see that ethers do not contain any acidic hydrogen and cannot give protons to Grignard’s reagent which is $C{{H}_{3}}MgBr$ in the given question.
Alcohols are acidic in nature due to the polar $O-H$ bond, therefore, can react with Grignard’s reagent $C{{H}_{3}}MgBr$ to give release ($C{{H}_{4}}$) gas. Reactions of all the four alcohols with $C{{H}_{3}}MgBr$ are given below:

Therefore, four out of the seven isomers of ${{C}_{4}}{{H}_{10}}O$ react with $C{{H}_{3}}MgBr$ to evolve $C{{H}_{4}}$ gas.
So, the correct answer is “Option B”.
Additional Information: One stereoisomer of butan-1-ol is possible as it has a chiral carbon. Stereoisomers have the same molecular and structural formula, i.e. have connectivity of atoms but different spatial arrangement of atoms.
Note: In order to react with $C{{H}_{3}}MgBr$, isomers of ${{C}_{4}}{{H}_{10}}O$ must have an acidic hydrogen. Ethers do not contain any acidic hydrogen as the electronegative O is connected to an alkyl chain, hence, they do not react with Grignard’s reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




