
How many isomers of $C_{4}H_{8}Cl_{2}$ are there? How do you tell if one structure is an isomer of another?
Answer
561k+ views
Hint: Isomers are compounds with the same molecular formula but different structural formulas and different properties and arrangement in space. Isomerism is the process of holding isomers and it can be classified into different types as structural, functional, stereo, and geometrical based on its structure, arrangement, and properties.
Complete step by step answer:
- Molecules or polyatomic ions with identical molecular formulas that have the same number of atoms of each element but distinct arrangements of atoms in space are known as isomers and the phenomenon is known as isomerism.
- In other words, the isomers in which the atoms are completely arranged in a different order with the same molecular formula is known as structural isomers.
- The compound given to us in the question is $C_{4}H_{8}Cl_{2}$ . There are possibly 13 isomers of the compound. In order to identify the isomers of a certain compound, one should write down the IUPAC name of the compound.
- Generally, a carbon atom has valency four, and thus, it forms four bonds with other atoms. The valency of chlorine is one and thus, it forms one bond with other atoms. The possible isomers for the compound are shown below:
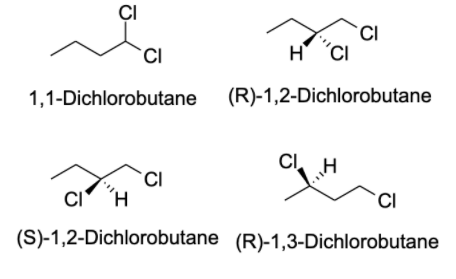
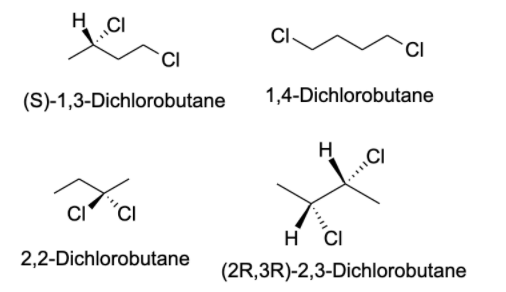
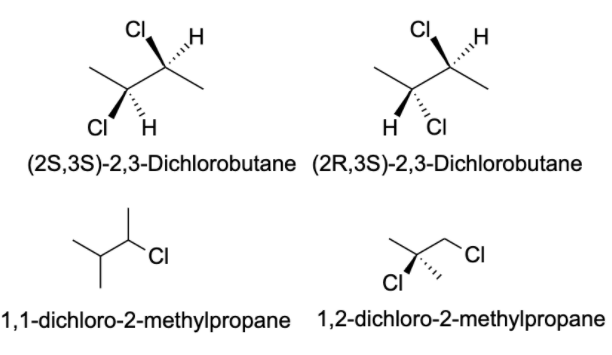
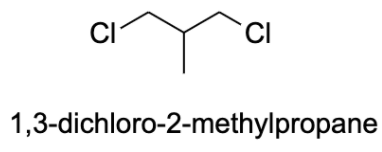
Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomer are present. Stereoisomerism is the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present.
Complete step by step answer:
- Molecules or polyatomic ions with identical molecular formulas that have the same number of atoms of each element but distinct arrangements of atoms in space are known as isomers and the phenomenon is known as isomerism.
- In other words, the isomers in which the atoms are completely arranged in a different order with the same molecular formula is known as structural isomers.
- The compound given to us in the question is $C_{4}H_{8}Cl_{2}$ . There are possibly 13 isomers of the compound. In order to identify the isomers of a certain compound, one should write down the IUPAC name of the compound.
- Generally, a carbon atom has valency four, and thus, it forms four bonds with other atoms. The valency of chlorine is one and thus, it forms one bond with other atoms. The possible isomers for the compound are shown below:




Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomer are present. Stereoisomerism is the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




