
Isopentane on monochlorination gives ____ isomer and out of them ___ are optically active:
A.3,1
B.4,2
C.3,2
D.4,1
Answer
564.3k+ views
Hint:Molecules having all the different groups on one carbon will be optically active. In monochlorination one hydrogen is replaced by the hydrogen.
Complete answer:
Monochlorination is the process of addition of one chlorine atom into the given molecule, mono represents one. Isomers are species which have different structures or arrangements but have the same molecular formulas. There are two types of isomers that are stereoisomer and structural isomers. The isomers formed by the isopentane are:
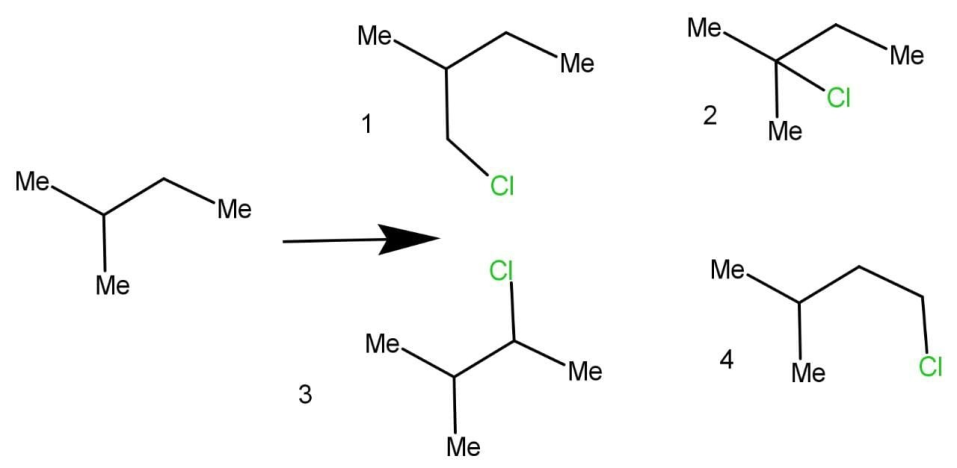
There are four isomers formed, as we can see that all have the same molecular formula. Optical activity arises due to rotation of plane polarised light by the molecule. The molecule either rotates the plane polarised right towards the right or towards the left. The one who rotates the light towards left is known as Laevo rotatory and the one who rotated the light toward right is known as dextro rotatory. There must be at least one chiral carbon for a molecule to be optically active. Chiral carbon is those carbon which have all four different groups attached to carbon. The structure 1 and 3 are optically active because of the presence of carbon with all different groups attached.
Hence, there are a total of 4 isomers and out of them 2 are optically active.
Thus, the correct option is B.
Note:
The optical activity can be checked by a POS that is point of symmetry and COS that is centre of symmetry. The molecules neither having POS nor COS are called optically active molecules.
Complete answer:
Monochlorination is the process of addition of one chlorine atom into the given molecule, mono represents one. Isomers are species which have different structures or arrangements but have the same molecular formulas. There are two types of isomers that are stereoisomer and structural isomers. The isomers formed by the isopentane are:

There are four isomers formed, as we can see that all have the same molecular formula. Optical activity arises due to rotation of plane polarised light by the molecule. The molecule either rotates the plane polarised right towards the right or towards the left. The one who rotates the light towards left is known as Laevo rotatory and the one who rotated the light toward right is known as dextro rotatory. There must be at least one chiral carbon for a molecule to be optically active. Chiral carbon is those carbon which have all four different groups attached to carbon. The structure 1 and 3 are optically active because of the presence of carbon with all different groups attached.
Hence, there are a total of 4 isomers and out of them 2 are optically active.
Thus, the correct option is B.
Note:
The optical activity can be checked by a POS that is point of symmetry and COS that is centre of symmetry. The molecules neither having POS nor COS are called optically active molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




