
Isopropylbenzene is oxidized in the presence of air to compound ‘A′. When compound ′A′ is treated with dilute mineral acid, the aromatic product formed is:
A. Phenol
B. Benzene
C. Benzaldehyde
D. Acetophenone
E. Toluene
Answer
583.8k+ views
Hint:
It is \[X \to \;A\; \to \;Y\] type of reaction. The major product will be A then the appropriate answer can be known. Here oxidize means the addition of the oxygen molecule. By adding oxygen to the organic molecule in the given compound the peroxide is formed, which is product A .it is further treated to give aromatic products which contain delocalized pi- electrons and other products will be non-aromatic ketone.
Complete step by step solution:
We can see that we have to find the first product A form isopropylbenzene, then after knowing A, the final product can be derived and the appropriate answer can be chosen. Here, we should not get confused with the product A but is taken as the intermediate product to derive the final product as shown in the figure the product A needs to be formed to get the aromatic product. The reactivity of cumene should be known and the structure of the compound will help to detect the tertiary carbon.
As shown in the figure the reaction is oxidation of cumene. Cumene is also known as Isopropyl benzene. Isopropyl benzene contains tertiary carbon to which the benzene ring is attached so the oxidation of it will give us product A which is cumene hydrogen peroxide. Further if it is treated with dilute mineral acid it will give us phenol as aromatic product and acetone as a by-product. In the given options, both the products are mentioned but the aromatic product as question specifies phenol,
Hence the correct answer is option (A).
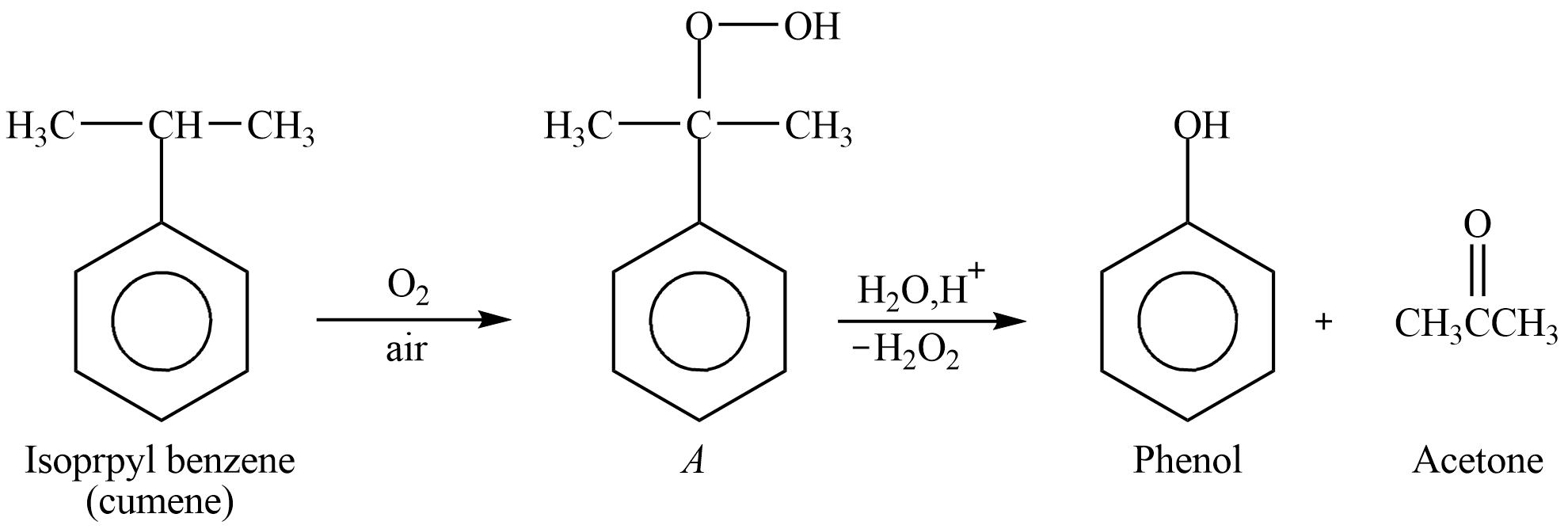
You may have a question why the oxygen attacks the tertiary carbon and no other carbon of the methyl group present at the neighboring carbon. The answer is that the tertiary carbocation is most stable, and hydrogen attached to it can be easily substituted by water molecule, carbon oxygen bond is formed in presence of acid. Here the role of adding acid is that, the bond between the tertiary carbon and carbon of the benzene breaks easily, so that hydroxyl group is attached to benzene aromatic ring and aliphatic ketone is formed with three carbon known as acetone or propanone, which is non-aromatic.
It is an important reaction as cumene hydrogen peroxide is used for the formation of the phenol and acetone.
Note:
Isopropylbenzene is also known as cumene. There are various reactions of cumene is possible. It is produced by Friedel crafts alkylation of benzene reacts with propylene. The use of cumene is to produce phenol and acetone.it is also used as a solvent in the paint industry.
It is \[X \to \;A\; \to \;Y\] type of reaction. The major product will be A then the appropriate answer can be known. Here oxidize means the addition of the oxygen molecule. By adding oxygen to the organic molecule in the given compound the peroxide is formed, which is product A .it is further treated to give aromatic products which contain delocalized pi- electrons and other products will be non-aromatic ketone.
Complete step by step solution:
We can see that we have to find the first product A form isopropylbenzene, then after knowing A, the final product can be derived and the appropriate answer can be chosen. Here, we should not get confused with the product A but is taken as the intermediate product to derive the final product as shown in the figure the product A needs to be formed to get the aromatic product. The reactivity of cumene should be known and the structure of the compound will help to detect the tertiary carbon.
As shown in the figure the reaction is oxidation of cumene. Cumene is also known as Isopropyl benzene. Isopropyl benzene contains tertiary carbon to which the benzene ring is attached so the oxidation of it will give us product A which is cumene hydrogen peroxide. Further if it is treated with dilute mineral acid it will give us phenol as aromatic product and acetone as a by-product. In the given options, both the products are mentioned but the aromatic product as question specifies phenol,
Hence the correct answer is option (A).

You may have a question why the oxygen attacks the tertiary carbon and no other carbon of the methyl group present at the neighboring carbon. The answer is that the tertiary carbocation is most stable, and hydrogen attached to it can be easily substituted by water molecule, carbon oxygen bond is formed in presence of acid. Here the role of adding acid is that, the bond between the tertiary carbon and carbon of the benzene breaks easily, so that hydroxyl group is attached to benzene aromatic ring and aliphatic ketone is formed with three carbon known as acetone or propanone, which is non-aromatic.
It is an important reaction as cumene hydrogen peroxide is used for the formation of the phenol and acetone.
Note:
Isopropylbenzene is also known as cumene. There are various reactions of cumene is possible. It is produced by Friedel crafts alkylation of benzene reacts with propylene. The use of cumene is to produce phenol and acetone.it is also used as a solvent in the paint industry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




