
IUPAC name of is:
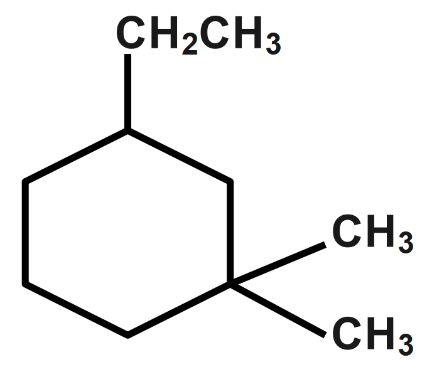
(A) $ 1,\text{ }1-dimethyl-3-ethyl\text{ }cyclohexane. $
(B) $ 3-Ethyl-1-dimethyl\;cyclohexane. $
(C) $ 3,\text{ }3-dimethyl-1-ethyl\text{ }cyclohexane. $
(D) $ 1-ethyl-3-3-dimethyl\text{ }cyclohexane. $

Answer
502.2k+ views
Hint :As we know, this IUPAC helped scientists to derive names of the molecules which were not even discovered. These rules have been revised from time to time to improve the quality of nomenclature.
Complete Step By Step Answer:
We know that, the parent hydrocarbon chain's carbon atoms must be counted using natural numbers, starting at the end and assigning the lowest number to the carbon atom that holds the substituents. The IUPAC stands for International Union of Pure and Applied Chemistry. It has formulated some rules for the systematic nomenclature of organic compounds which are even revised later on for the uniform naming of compounds. The IUPAC has laid down some rules which govern the naming of all the organic compounds. This is necessary so that there is uniform nomenclature to be followed world-wide.
Now, let's consider different options:
- $ 1,\text{ }1-dimethyl-3-ethyl\text{ }cyclohexane $ : This option is not correct as two carbon compounds should be called ethyl, not as $ 1,1-dimethyl. $ Hence the option is incorrect.
- $ 3-Ethyl-1-dimethyl\;cyclohexane $ : This is a correct option as we start the counting from the ethyl end. Hence, the option is correct.
- $ 3,\text{ }3-dimethyl-1-ethyl\text{ }cyclohexane $ : There is no three dimethyl in the third position and hence the answer is incorrect. This naming is also incorrect.
- $ 1-ethyl-3-3-dimethyl\text{ }cyclohexane $ : This is a correct option as we start the counting from the di methyl end. Hence, the option is correct.
Thus, the given compound can have two different IUPAC names according to the counting.
Therefore, the correct options are B and D.
Note :
Remember that the IUPAC nomenclature is needed because earlier every chemist gave its own new name to the molecule it discovered. As a result, one molecule had different names at different places. It was difficult to study them. No one knows whether the molecule discovered by him/her is the new one or the one that has already been discovered.
Complete Step By Step Answer:
We know that, the parent hydrocarbon chain's carbon atoms must be counted using natural numbers, starting at the end and assigning the lowest number to the carbon atom that holds the substituents. The IUPAC stands for International Union of Pure and Applied Chemistry. It has formulated some rules for the systematic nomenclature of organic compounds which are even revised later on for the uniform naming of compounds. The IUPAC has laid down some rules which govern the naming of all the organic compounds. This is necessary so that there is uniform nomenclature to be followed world-wide.
Now, let's consider different options:
- $ 1,\text{ }1-dimethyl-3-ethyl\text{ }cyclohexane $ : This option is not correct as two carbon compounds should be called ethyl, not as $ 1,1-dimethyl. $ Hence the option is incorrect.
- $ 3-Ethyl-1-dimethyl\;cyclohexane $ : This is a correct option as we start the counting from the ethyl end. Hence, the option is correct.
- $ 3,\text{ }3-dimethyl-1-ethyl\text{ }cyclohexane $ : There is no three dimethyl in the third position and hence the answer is incorrect. This naming is also incorrect.
- $ 1-ethyl-3-3-dimethyl\text{ }cyclohexane $ : This is a correct option as we start the counting from the di methyl end. Hence, the option is correct.
Thus, the given compound can have two different IUPAC names according to the counting.
Therefore, the correct options are B and D.
Note :
Remember that the IUPAC nomenclature is needed because earlier every chemist gave its own new name to the molecule it discovered. As a result, one molecule had different names at different places. It was difficult to study them. No one knows whether the molecule discovered by him/her is the new one or the one that has already been discovered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




