
What is the IUPAC name of Iso-octane?
A) Octane
B) \[2,2,4 - Trimethylpentane\]
C) \[2,5 - Dimethylhexane\]
D) \[2 - methylheptane\]
Answer
524.4k+ views
Hint: We have to remember that the isoforms are the simplest form of isomers having all carbons in a straight chain or branched if a chain is long, except for one carbon which restricts the continuation of carbon atom in a straight or branched chain. For example:
Iso-butane, Iso-pentane.
Complete step by step answer:
We have to remember that an octane is a hydrocarbon having a chemical formula C8H18. It comes under the class of alkane as it has all single C-H bonds.
There are many isomers of octane which differ in the structural formula or you can say the position of Carbon atoms.
Option A) this is an incorrect option as octane is not an iso structure of octane.
Octane chemical formula is C8H18
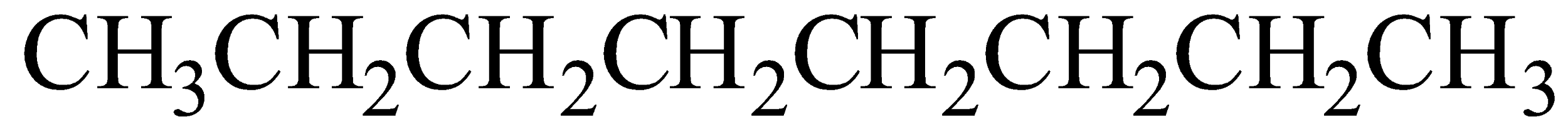
Option B) This is a correct option as \[2,2,4 - Trimethylpentane\] is IUPAC name of iso-octane.
Structure of iso octane is as follows:
\[{\left( {C{H_3}} \right)_3}CC{H_2}{\left( {C{H_3}} \right)_2}\] or
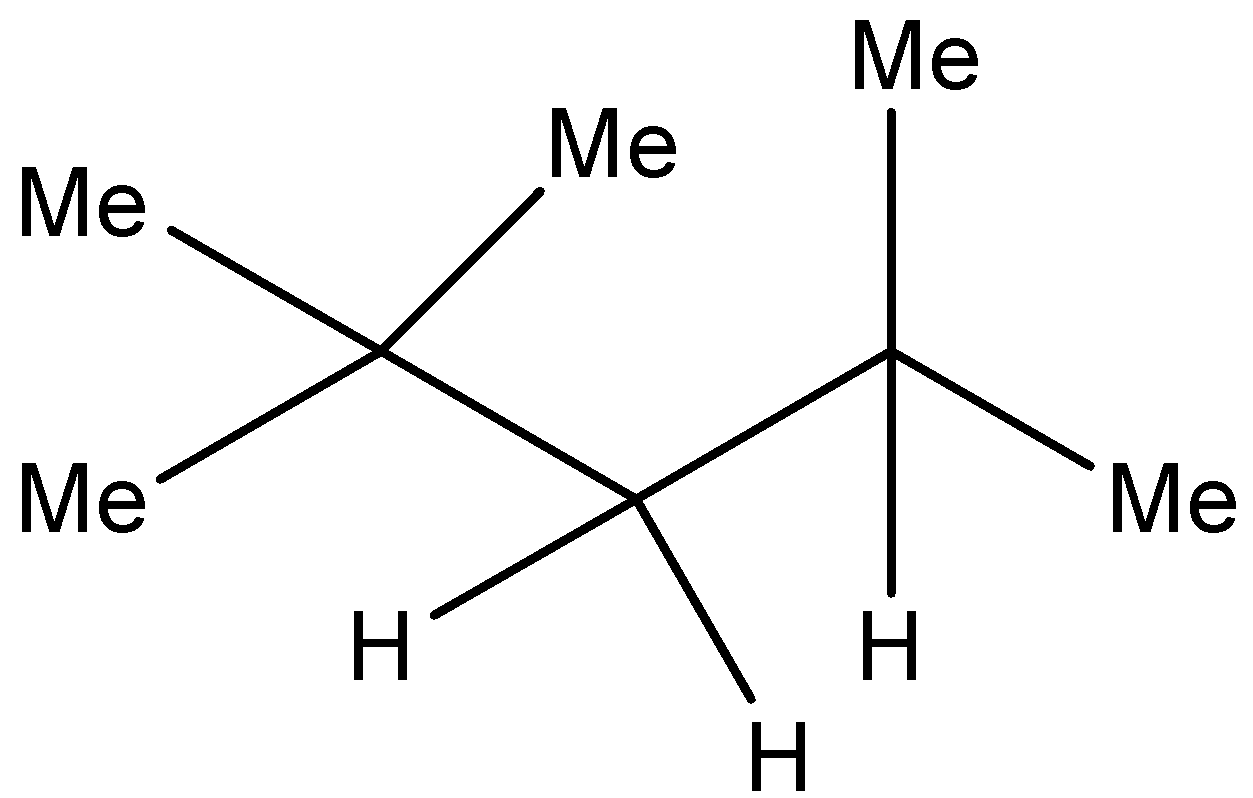
This is the most common isomer of octane. This chemical is used to obtain the octane rating of any fuel.
Octane is less dense than water and therefore is insoluble in it. Also octane is a component of gasoline.
Option C) this is an incorrect option as \[2,5 - Dimethylhexane\]is an isomer of octane but it does not belong to iso structure.
The structural formula for \[2,5 - Dimethylhexane\] is as follows:
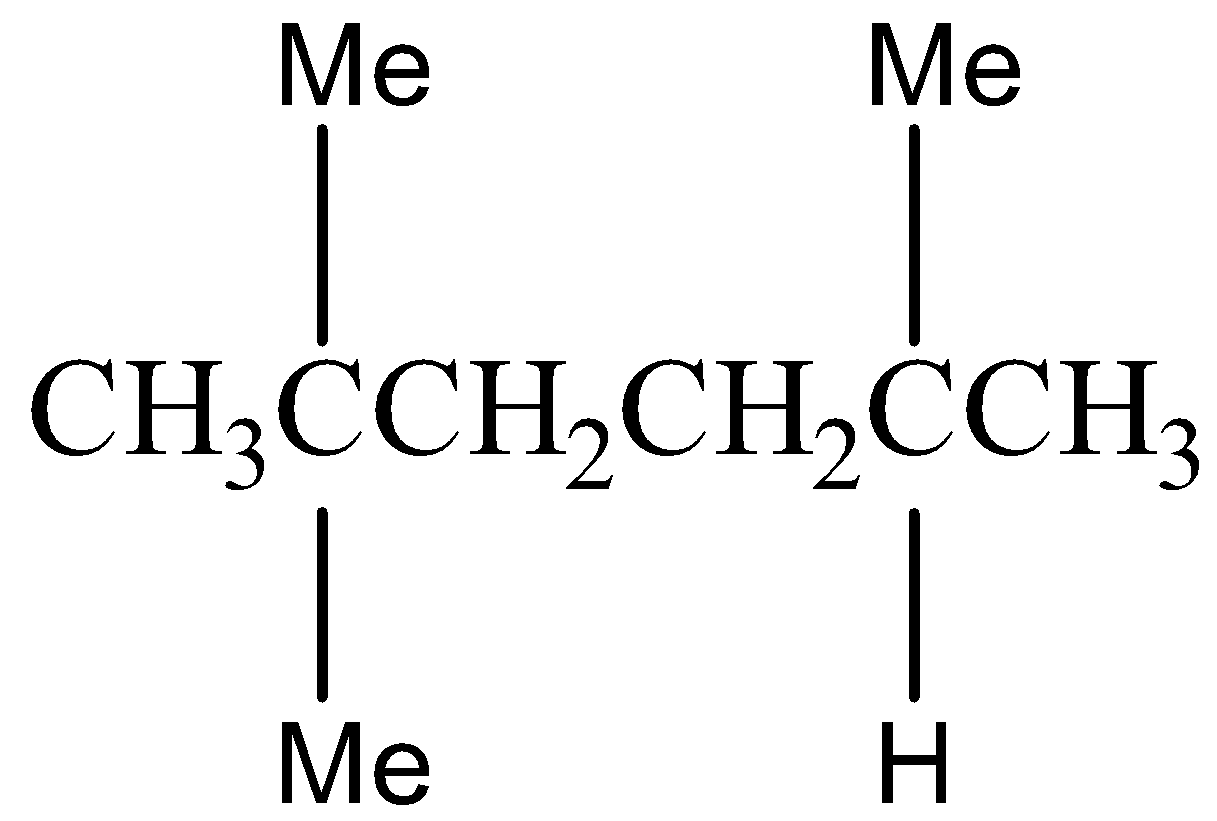
Option D) this is an incorrect option as \[2 - methylheptane\] is an isomer of octane but it does not belong to iso isomer.
Structural formula of \[2 - methylheptane\] is as follows:

Note: We have to remember that the octane has several isomers that are structural isomers but the most common in it is iso-octane having a chemical name as \[2,2,4 - Trimethylpentane\].
Iso-butane, Iso-pentane.
Complete step by step answer:
We have to remember that an octane is a hydrocarbon having a chemical formula C8H18. It comes under the class of alkane as it has all single C-H bonds.
There are many isomers of octane which differ in the structural formula or you can say the position of Carbon atoms.
Option A) this is an incorrect option as octane is not an iso structure of octane.
Octane chemical formula is C8H18
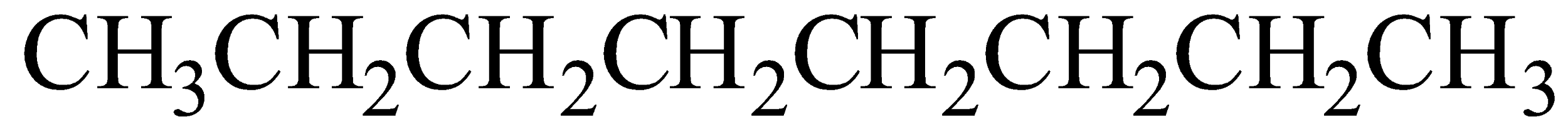
Option B) This is a correct option as \[2,2,4 - Trimethylpentane\] is IUPAC name of iso-octane.
Structure of iso octane is as follows:
\[{\left( {C{H_3}} \right)_3}CC{H_2}{\left( {C{H_3}} \right)_2}\] or

This is the most common isomer of octane. This chemical is used to obtain the octane rating of any fuel.
Octane is less dense than water and therefore is insoluble in it. Also octane is a component of gasoline.
Option C) this is an incorrect option as \[2,5 - Dimethylhexane\]is an isomer of octane but it does not belong to iso structure.
The structural formula for \[2,5 - Dimethylhexane\] is as follows:

Option D) this is an incorrect option as \[2 - methylheptane\] is an isomer of octane but it does not belong to iso isomer.
Structural formula of \[2 - methylheptane\] is as follows:

Note: We have to remember that the octane has several isomers that are structural isomers but the most common in it is iso-octane having a chemical name as \[2,2,4 - Trimethylpentane\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




