
IUPAC name of the compound is
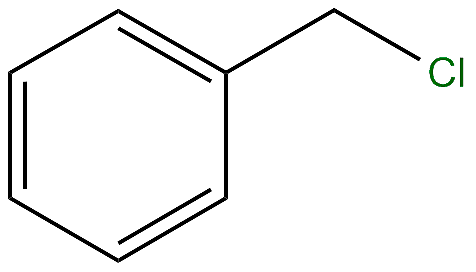
(A) Chloromethyl benzene
(B) Chlorophenyl benzene
(C) Both A and B
(D) None of the above

Answer
514.2k+ views
Hint :The IUPAC nomenclature system is a set of logical guidelines developed and utilised by organic chemists to avoid the issues that arbitrary naming may generate. Knowing these guidelines and being provided a structural formula, one should be able to write a separate name for each compound. Similarly, given an IUPAC name, a structural formula should be able to be written. An IUPAC name will typically contain three components: a root, a suffix, and a functional group.
Complete Step By Step Answer:
Substituted benzene ring compounds have a less systematic nomenclature than alkanes, alkenes, and alkynes. The bulk of these compounds, on the other hand, have one-of-a-kind names. In order to learn these names, memorising is the only option.
Phenyl, abbreviated Ph-, and benzyl, abbreviated Bn-, are two often encountered substituent groups that integrate a benzene ring. A phenyl group should not be confused with the chemical phenol. Ar- for an aryl group is a broad and helpful generic notation that complements the usage of R- for an alkyl group (any aromatic ring).
When there are many substituents on a benzene ring, the relative positions of the substituents must be indicated by numbering the ring carbons or by some other method. The prefixes ortho, meta, and para are widely employed to signify a 1,2-, 1,3-, or 1,4- connection in the case of disubstituted benzenes. The first row of compounds in the following examples shows this use in red. Some substituted toluenes have singular names (for example, xylene, cresol, and toluidine), and their isomers are usually labelled with the ortho, meta, or para prefix.
Here the substituent in the given compound is chloromethane (prefix).
Hence the answer is Option A .
Note :
An alkylating agent is benzoyl chloride. In a hydrolysis process, benzyl chloride combines with water to generate benzyl alcohol and hydrochloric acid, indicating its high reactivity (compared to alkyl chlorides). Hydrolysis releases hydrochloric acid when it comes into touch with mucosal membranes. As a lachrymator, benzyl chloride has been employed in chemical warfare. It also has a strong irritant effect on the skin.
Complete Step By Step Answer:
Substituted benzene ring compounds have a less systematic nomenclature than alkanes, alkenes, and alkynes. The bulk of these compounds, on the other hand, have one-of-a-kind names. In order to learn these names, memorising is the only option.
Phenyl, abbreviated Ph-, and benzyl, abbreviated Bn-, are two often encountered substituent groups that integrate a benzene ring. A phenyl group should not be confused with the chemical phenol. Ar- for an aryl group is a broad and helpful generic notation that complements the usage of R- for an alkyl group (any aromatic ring).
When there are many substituents on a benzene ring, the relative positions of the substituents must be indicated by numbering the ring carbons or by some other method. The prefixes ortho, meta, and para are widely employed to signify a 1,2-, 1,3-, or 1,4- connection in the case of disubstituted benzenes. The first row of compounds in the following examples shows this use in red. Some substituted toluenes have singular names (for example, xylene, cresol, and toluidine), and their isomers are usually labelled with the ortho, meta, or para prefix.
Here the substituent in the given compound is chloromethane (prefix).
Hence the answer is Option A .
Note :
An alkylating agent is benzoyl chloride. In a hydrolysis process, benzyl chloride combines with water to generate benzyl alcohol and hydrochloric acid, indicating its high reactivity (compared to alkyl chlorides). Hydrolysis releases hydrochloric acid when it comes into touch with mucosal membranes. As a lachrymator, benzyl chloride has been employed in chemical warfare. It also has a strong irritant effect on the skin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




