
J.J.Thomson’s cathode ray tube experiment demonstrated that:
A. Cathode rays are streams of negatively charged ions
B. All the mass of an atom is essentially in the nucleus
C. The $\dfrac{e}{m}$ of electrons is much greater than the $\dfrac{e}{m}$ of protons.
D. The $\dfrac{e}{m}$ ratio of the cathode ray particles changes when a different gas is placed in the discharged tube.
Answer
553.8k+ views
Hint: J.J Thomson’s is known for Ws famous experiment cathode ray tube which should know that all atoms contain tiny negatively charged subatomic particles or electrons. He also proposed that direction of ratio of $e/m$ vary with charge of use of cathode ray and proton.
Complete step by step answer:
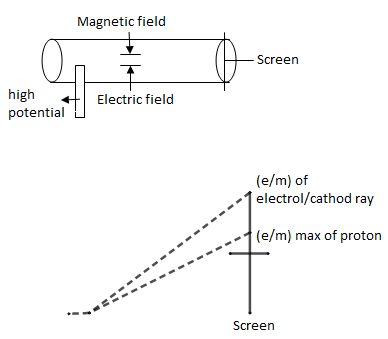
As we all know beforehand, the theory of J.J Thomson’s on atom, the john Dalton proposed his atomic theory in 1808. According to him all matter on the earth is composed of small, hard, invisible particles called atoms. Elements in atoms are identical and have a characteristic mass. Dalton said that atoms combine to form a molecule which is the smallest unit of a compound.
Later it was believed that atoms were invisible until J.J Thomson discovered the electron in 1897. Thomson proposed his atomic model in 1903. According to him, an atom consists of a positively charged sphere with negatively charged electrons embedded in it. The number of electrons in an atom are such that the atom, as a whole, has zero charge.
From the above statement we can say that emission of negatively charged particles from elements indicated that atoms could be split into positive and negative charged particles and atoms possess some internal structure.
In this experiment we had an tube at one end of it, high potential has attached because of which ${{e}^{-}}$ emitted and because of presence of magnets and electric field in tube, we saw deflection on screen, Because of this deflection only, ratio of $e/m$ on screen is a proton. But when he performed with cathode ray, deflection was more & screen went up. This way the ratio of $e/m$ of ${{e}^{-}}$ is much greater than $e/m$ of protons.
Therefore, option (A) and (C) are correct options.
Note: Thomson could not explain the stability of structure of atom and atomic spectra. Also he couldn’t show that all the mass contained in atoms is because of the mass of the nucleus only. Therefore, the statement given in option (B) is wrong. Change of ratio of $e/m$ cathode ray particles are independent of changes in gas which is plaid in the discharged tube.
Complete step by step answer:

As we all know beforehand, the theory of J.J Thomson’s on atom, the john Dalton proposed his atomic theory in 1808. According to him all matter on the earth is composed of small, hard, invisible particles called atoms. Elements in atoms are identical and have a characteristic mass. Dalton said that atoms combine to form a molecule which is the smallest unit of a compound.
Later it was believed that atoms were invisible until J.J Thomson discovered the electron in 1897. Thomson proposed his atomic model in 1903. According to him, an atom consists of a positively charged sphere with negatively charged electrons embedded in it. The number of electrons in an atom are such that the atom, as a whole, has zero charge.
From the above statement we can say that emission of negatively charged particles from elements indicated that atoms could be split into positive and negative charged particles and atoms possess some internal structure.
In this experiment we had an tube at one end of it, high potential has attached because of which ${{e}^{-}}$ emitted and because of presence of magnets and electric field in tube, we saw deflection on screen, Because of this deflection only, ratio of $e/m$ on screen is a proton. But when he performed with cathode ray, deflection was more & screen went up. This way the ratio of $e/m$ of ${{e}^{-}}$ is much greater than $e/m$ of protons.
Therefore, option (A) and (C) are correct options.
Note: Thomson could not explain the stability of structure of atom and atomic spectra. Also he couldn’t show that all the mass contained in atoms is because of the mass of the nucleus only. Therefore, the statement given in option (B) is wrong. Change of ratio of $e/m$ cathode ray particles are independent of changes in gas which is plaid in the discharged tube.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




