
How do you know the number of electrons in the outermost energy level of an atom?
Answer
558.6k+ views
Hint: The electrons encompassing an atom are situated in areas around the nucleus called "energy levels". An energy level speaks to the \[3\]-dimensional space encompassing the nucleus where electrons are most likely to be.
Complete solution:
The principal energy level is nearest to the nucleus. The subsequent energy level is somewhat farther away than the first. The third is somewhat farther away than the second, etc. Every energy level can oblige or "hold" an alternate number of electrons before extra electrons start to go into the following level. At the point when the principal energy level has \[2\] electrons, the following electrons go into the subsequent energy level until the subsequent level has \[8\] electrons. At the point when the subsequent energy level has \[8\] electrons, the following electrons go into the third energy level until the third level has \[8\] electrons. At the moment once the third energy has eight electrons, the subsequent pair of electrons get into the fourth energy. The electrons within the energy level farthest from the nucleus are known as valence electrons. Atoms in a similar column in the occasional table has a similar number of valence electrons.
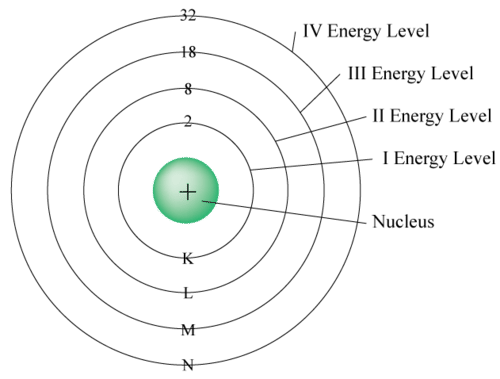
Note: In the event that the energy of a molecule is expanded, an electron in the particle gets energized. To return to its ground state, the electron discharges energy. The energy of the light delivered when an electron drops in energy level is equivalent to the distinction in energy between the two levels.
Electrons are located in shells around an atom nucleus. Electrons nearest to the core will have the most reduced energy. Electrons further away from the core will have higher energy. A molecule's electron shell can oblige \[2{n^2}\] electrons (where n is the shell level).
Complete solution:
The principal energy level is nearest to the nucleus. The subsequent energy level is somewhat farther away than the first. The third is somewhat farther away than the second, etc. Every energy level can oblige or "hold" an alternate number of electrons before extra electrons start to go into the following level. At the point when the principal energy level has \[2\] electrons, the following electrons go into the subsequent energy level until the subsequent level has \[8\] electrons. At the point when the subsequent energy level has \[8\] electrons, the following electrons go into the third energy level until the third level has \[8\] electrons. At the moment once the third energy has eight electrons, the subsequent pair of electrons get into the fourth energy. The electrons within the energy level farthest from the nucleus are known as valence electrons. Atoms in a similar column in the occasional table has a similar number of valence electrons.

Note: In the event that the energy of a molecule is expanded, an electron in the particle gets energized. To return to its ground state, the electron discharges energy. The energy of the light delivered when an electron drops in energy level is equivalent to the distinction in energy between the two levels.
Electrons are located in shells around an atom nucleus. Electrons nearest to the core will have the most reduced energy. Electrons further away from the core will have higher energy. A molecule's electron shell can oblige \[2{n^2}\] electrons (where n is the shell level).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




