
${{\Lambda }^{\infty }}$of strong electrolytes can be obtained by extrapolating $\Lambda $vs $\sqrt{c}$curves to 0 concentration. If true enter 1 else 0.
Answer
588.6k+ views
Hint: Molar conductivity is the conductance property of a solution that contains exactly one mole of the electrolyte or it the function of the ionic strength of a solution or the concentration of the salt present in the solution.
Complete Step by step solution:
-The conducting power of all the ions produced by dissolving one mole of an electrolyte in solution is known as molar conductivity.
-Molar conductivity is represented by the lambda, that is $'\Lambda '$ .
-Molar conductivity is mathematically the conductivity of the solution divided by its molar concentration, that is,
${{\Lambda }_{m}}=\dfrac{k}{c}$
where k = the measured conductivity or the specific conductance
c = molar concentration of the electrolyte
-We can also define the molar conductivity of an electrolyte as the conductance of the electrolyte per volume containing a unit mole of the electrolyte which is placed between two electrodes of unit area cross-section or at a distance of 1cm apart.
-Siemens meter-squared per mole $(S{{m}^{2}}mo{{l}^{-1}})$ the SI unit of molar conductivity.
-For both weak and strong electrolytes, the molar conductivity increases with the decrease in the concentration or with the dilution of the electrolyte. As the increased dilutions lead to the more dissociation of electrolytes which subsequently increases the number of active ions in the solutions and thereby increasing the conductivity.
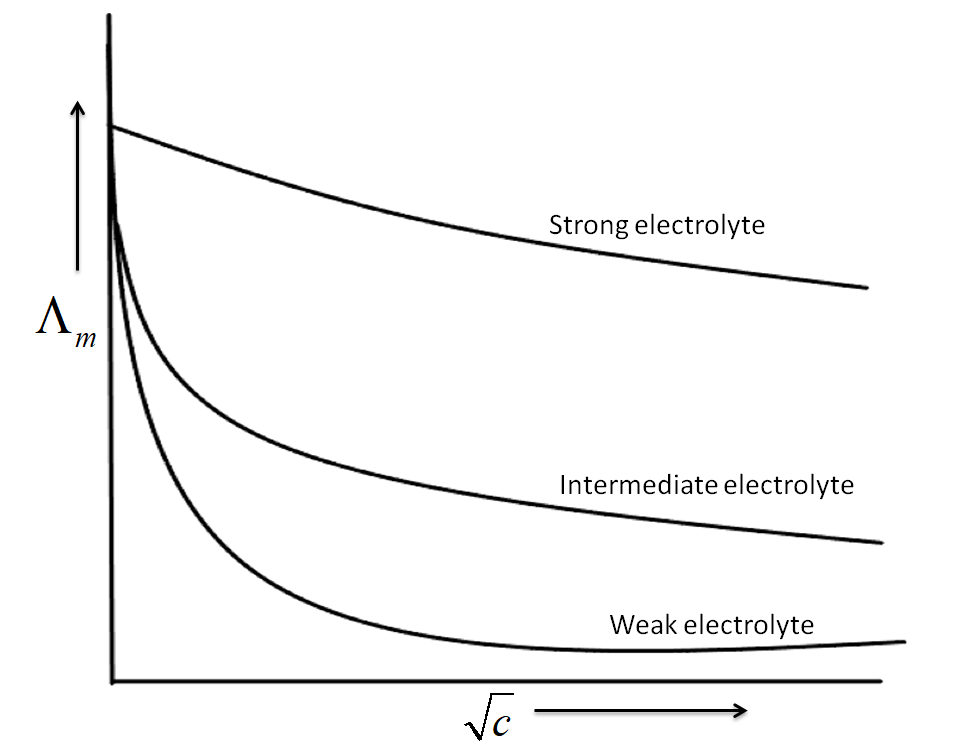
The graph above clearly shows that for strong electrolytes, the molar conductivity increases slowly with the dilution.
-It ${{E}^{{}^\circ }}$ is the molar conductivity at zero concentration, that is the limiting molar conductivity, the general equation for the plot of the strong electrolyte is given by the Kohlrausch’s law, that is
${{\Lambda }_{m}}=E{}^\circ m-A\sqrt{c}$
where A represents the slope of the plot and depends on the type of electrolyte at a given temperature for a given solvent.
-For strong electrolytes, the increase in the concentration of the electrolyte produces a sharp increase in the conductivity, but at lower concentrations, weak electrolytes give very low values for the specific conductivity, which gradually increases with the increase in concentrations.
Note: The dissociation constant of a weak electrolyte as a function of concentration which is given by Ostwald’s law of dilution is an application of the Molar conductivity, as the dissociation constant can be written in terms of molar conductivity.
Complete Step by step solution:
-The conducting power of all the ions produced by dissolving one mole of an electrolyte in solution is known as molar conductivity.
-Molar conductivity is represented by the lambda, that is $'\Lambda '$ .
-Molar conductivity is mathematically the conductivity of the solution divided by its molar concentration, that is,
${{\Lambda }_{m}}=\dfrac{k}{c}$
where k = the measured conductivity or the specific conductance
c = molar concentration of the electrolyte
-We can also define the molar conductivity of an electrolyte as the conductance of the electrolyte per volume containing a unit mole of the electrolyte which is placed between two electrodes of unit area cross-section or at a distance of 1cm apart.
-Siemens meter-squared per mole $(S{{m}^{2}}mo{{l}^{-1}})$ the SI unit of molar conductivity.
-For both weak and strong electrolytes, the molar conductivity increases with the decrease in the concentration or with the dilution of the electrolyte. As the increased dilutions lead to the more dissociation of electrolytes which subsequently increases the number of active ions in the solutions and thereby increasing the conductivity.

The graph above clearly shows that for strong electrolytes, the molar conductivity increases slowly with the dilution.
-It ${{E}^{{}^\circ }}$ is the molar conductivity at zero concentration, that is the limiting molar conductivity, the general equation for the plot of the strong electrolyte is given by the Kohlrausch’s law, that is
${{\Lambda }_{m}}=E{}^\circ m-A\sqrt{c}$
where A represents the slope of the plot and depends on the type of electrolyte at a given temperature for a given solvent.
-For strong electrolytes, the increase in the concentration of the electrolyte produces a sharp increase in the conductivity, but at lower concentrations, weak electrolytes give very low values for the specific conductivity, which gradually increases with the increase in concentrations.
Note: The dissociation constant of a weak electrolyte as a function of concentration which is given by Ostwald’s law of dilution is an application of the Molar conductivity, as the dissociation constant can be written in terms of molar conductivity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




