
What is the law of conservation of energy states?
Answer
495.6k+ views
Hint: The evolution of living forms on Earth necessitates the use of energy. It is defined as the ability to perform work in physics. We know that energy occurs in nature in various forms. You've learned about several types of energy, such as heat, electricity, chemical energy, nuclear energy, and so on. We will study the rules and concepts that control energy in this essay. The law of conservation of energy is the name given to this rule.
Complete answer:
Law of conservation of energy states that energy cannot be created or destroyed. The transfer of energy from one body to another is feasible.
In other words, the Law of Conservation of Energy states that the amount of energy exiting before an event is equivalent to the amount of energy after the event.
Law of conservation of energy:
Energy cannot be created or destroyed, according to the rule of conservation of energy. It can, however, be changed from one formation to another. The overall energy of an isolated system remains constant when all forms of energy are considered. All types of energy are subject to the law of conservation of energy. In a nutshell, the rule of conservation of energy asserts that the total energy of a closed system, that is, one that is separated from its surroundings, is conserved.
If there is a loss of energy in one area of an isolated system like the cosmos, there must be a gain of energy in another section of the universe. There is no known cause of the principle of energy conservation being violated, even though it cannot be proved.
The below equation determines the amount of energy in any system:
${U_T} = {U_i} + W + Q$
${U_T}$ - Total energy of a system
${U_i}$ - Initial energy of a system
$W$ - Heat is introduced into the system or removed from it.
$Q$ - Work carried out by or on behalf of the system.
The equation is used to calculate the change in the system's internal energy.
$\Delta U = W + Q$
Note:
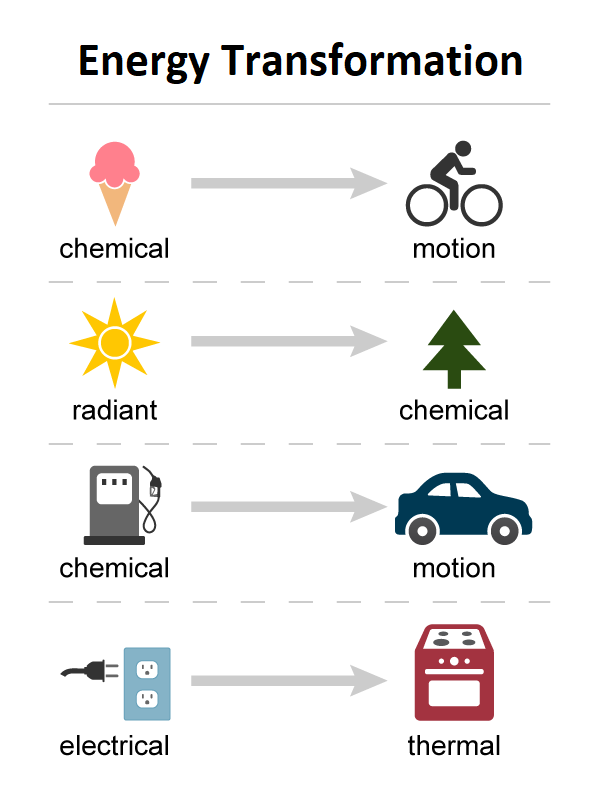
Examples of the Energy Conservation Law:
A torch converts the chemical energy of the batteries into electrical energy, which is then transferred into light and heat.
Waterfalls from a height on the turbines in hydroelectric power facilities. This causes the turbines to spin and generate power. As a result, water's potential energy is turned into the turbine's kinetic energy, which is then converted into electrical energy.
Electrical energy is turned into sound energy in a loudspeaker.
Sound energy is turned into electrical energy in a microphone.
Chemical energy is transformed into heat and light energy when fuels are burned.
Complete answer:
Law of conservation of energy states that energy cannot be created or destroyed. The transfer of energy from one body to another is feasible.
In other words, the Law of Conservation of Energy states that the amount of energy exiting before an event is equivalent to the amount of energy after the event.
Law of conservation of energy:
Energy cannot be created or destroyed, according to the rule of conservation of energy. It can, however, be changed from one formation to another. The overall energy of an isolated system remains constant when all forms of energy are considered. All types of energy are subject to the law of conservation of energy. In a nutshell, the rule of conservation of energy asserts that the total energy of a closed system, that is, one that is separated from its surroundings, is conserved.

If there is a loss of energy in one area of an isolated system like the cosmos, there must be a gain of energy in another section of the universe. There is no known cause of the principle of energy conservation being violated, even though it cannot be proved.
The below equation determines the amount of energy in any system:
${U_T} = {U_i} + W + Q$
${U_T}$ - Total energy of a system
${U_i}$ - Initial energy of a system
$W$ - Heat is introduced into the system or removed from it.
$Q$ - Work carried out by or on behalf of the system.
The equation is used to calculate the change in the system's internal energy.
$\Delta U = W + Q$
Note:
Examples of the Energy Conservation Law:
A torch converts the chemical energy of the batteries into electrical energy, which is then transferred into light and heat.
Waterfalls from a height on the turbines in hydroelectric power facilities. This causes the turbines to spin and generate power. As a result, water's potential energy is turned into the turbine's kinetic energy, which is then converted into electrical energy.
Electrical energy is turned into sound energy in a loudspeaker.
Sound energy is turned into electrical energy in a microphone.
Chemical energy is transformed into heat and light energy when fuels are burned.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




