
When lead accumulator is charged, it acts as-
A.An electrolytic cell
B.A galvanic cell
C.A Daniel cell
D.None of the above
Answer
591.3k+ views
Hint: When electrical energy is converted into chemical energy by applying an external source it is known as charging in lead accumulator. Lead accumulator is a secondary cell.
Complete step by step answer:
-Lead accumulator is a secondary cell. They are repeated action cells. They can be recharged by passing electricity through them after they discharge. So the secondary cell can be used for a long period of time. These cells are called storage cells. The cells are rechargeable as the electrode reactions are reversible and the process can be repeated many times.
-When the electrical energy is converted into chemical energy by applying an external source then the lead accumulator is said to be charging. This starts non-spontaneous chemical reactions. So when a charging lead accumulator acts as an electrolytic cell because the electrolytic cell converts electrical energy into chemical energy.
-In this process the sulphuric acid is regenerated which acts as an electrolyte so the cells can be used again and again by regenerating the sulphuric acid.
Hence the correct answer is A.
Additional Information:
- In lead accumulator, the anode and the cathode plates are arranged alternately and separated from each other by separators. The plates are connected in parallel. This increases the surface area and the current producing capacity of the cell. See the diagram below-
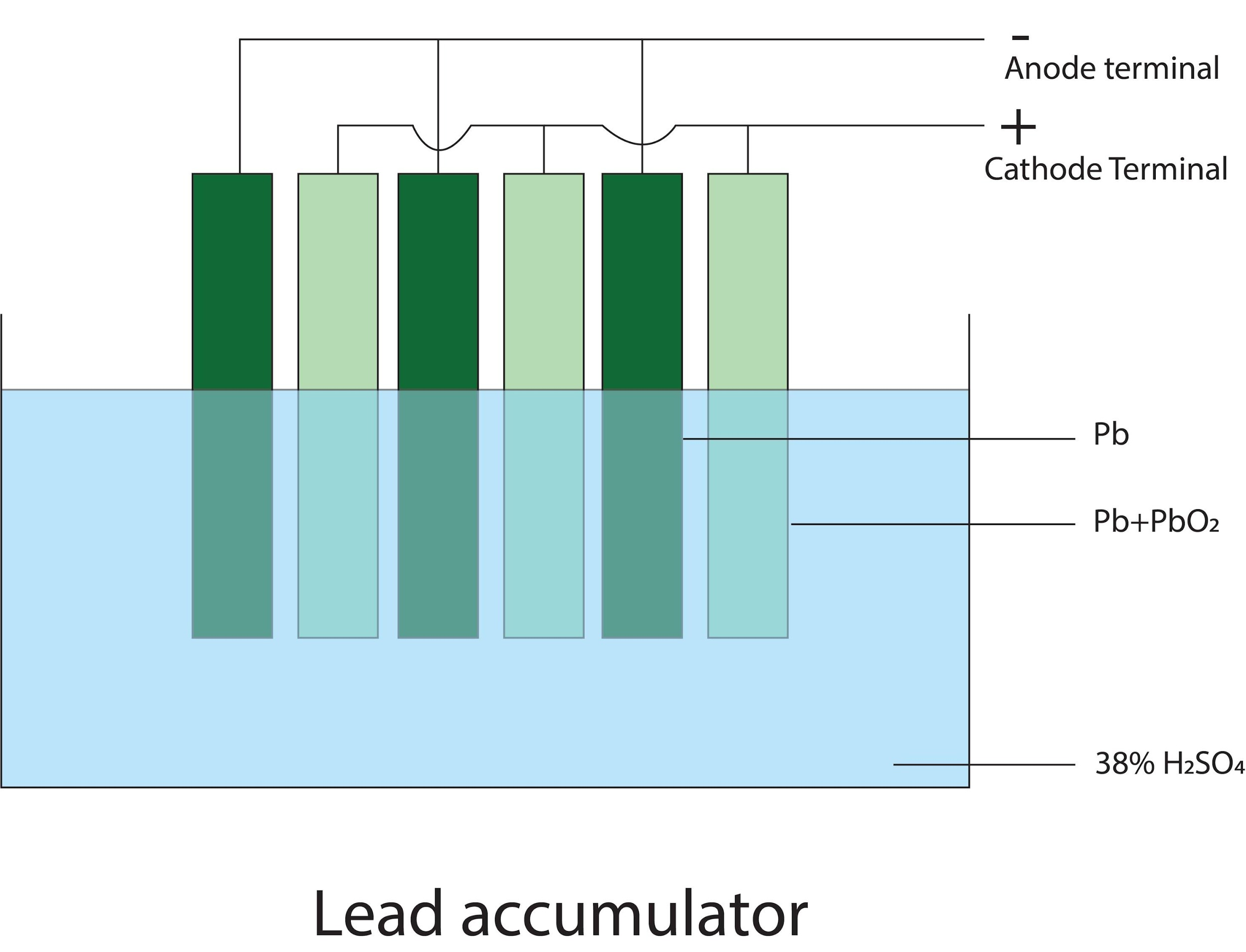
-The Cathode plate is coated with lead oxide and anode with lead and both are dissolved in dil. Sulphuric acid. When the cell is discharged the lead cell is coated with white precipitate of lead sulphate which stops the reaction. So when a current is supplied the lead sulphate converts back to lead and lead oxide on the respective electrode/plates. This is how the lead accumulator works.
Note:
Lead accumulator has many applications-
-It is used in motor cars and other automotive vehicles. It is also known as a car battery.
-It is used in the laboratory as a source of constant DC voltage.
-It is used in telephone and telegraph offices.
-It is used in electric clocks and radio sets.
-It is used as a source of electricity in rockets in initial stages.
Complete step by step answer:
-Lead accumulator is a secondary cell. They are repeated action cells. They can be recharged by passing electricity through them after they discharge. So the secondary cell can be used for a long period of time. These cells are called storage cells. The cells are rechargeable as the electrode reactions are reversible and the process can be repeated many times.
-When the electrical energy is converted into chemical energy by applying an external source then the lead accumulator is said to be charging. This starts non-spontaneous chemical reactions. So when a charging lead accumulator acts as an electrolytic cell because the electrolytic cell converts electrical energy into chemical energy.
-In this process the sulphuric acid is regenerated which acts as an electrolyte so the cells can be used again and again by regenerating the sulphuric acid.
Hence the correct answer is A.
Additional Information:
- In lead accumulator, the anode and the cathode plates are arranged alternately and separated from each other by separators. The plates are connected in parallel. This increases the surface area and the current producing capacity of the cell. See the diagram below-

-The Cathode plate is coated with lead oxide and anode with lead and both are dissolved in dil. Sulphuric acid. When the cell is discharged the lead cell is coated with white precipitate of lead sulphate which stops the reaction. So when a current is supplied the lead sulphate converts back to lead and lead oxide on the respective electrode/plates. This is how the lead accumulator works.
Note:
Lead accumulator has many applications-
-It is used in motor cars and other automotive vehicles. It is also known as a car battery.
-It is used in the laboratory as a source of constant DC voltage.
-It is used in telephone and telegraph offices.
-It is used in electric clocks and radio sets.
-It is used as a source of electricity in rockets in initial stages.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




