
${{\left[ Si{{O}_{4}} \right]}^{4-}}$ has tetrahedral structure, the silicate formed using three oxygen has
(A) Two dimensional sheet structure
(B) Pyrosilicate structure
(C) Linear polymeric structure
(d) Three dimensional structure
Answer
588.6k+ views
Solution:
Hint: VSEPR theory states that the electron pairs repel each other, whether they are in the form of lone pair or bond pair. This is done in order to minimise the repulsion, thus increasing the stability of the compound.
Complete step by step solution:
-To draw the structure of any molecule, we must know the group of the atoms present in the molecule. The groups tell us about the valence electrons of the atom. This in turn gives us the number of bonds needed to be connected to the atom to complete its octet. If there are less number of bonds for an atom, then we represent it with a negative sign.
-To talk about ${{\left[ Si{{O}_{4}} \right]}^{4-}}$
Here we see that silicon is the centrala tom and it lies in group 4 while oxygen in group 16. So, silicon should have 4 bonds while oxygen should have 2 bonds. Now as there are 4 oxygen atoms, we link those to silicon to complete the octet of silicon. Since there are no more atoms, the octet of oxygen atoms is not complete and so they are represented by a negative sign. Thus, the total charge present on the molecule is -4.
-Since there are 4 bonds which are all identical, the shape of this compound will be tetrahedral and can be shown as
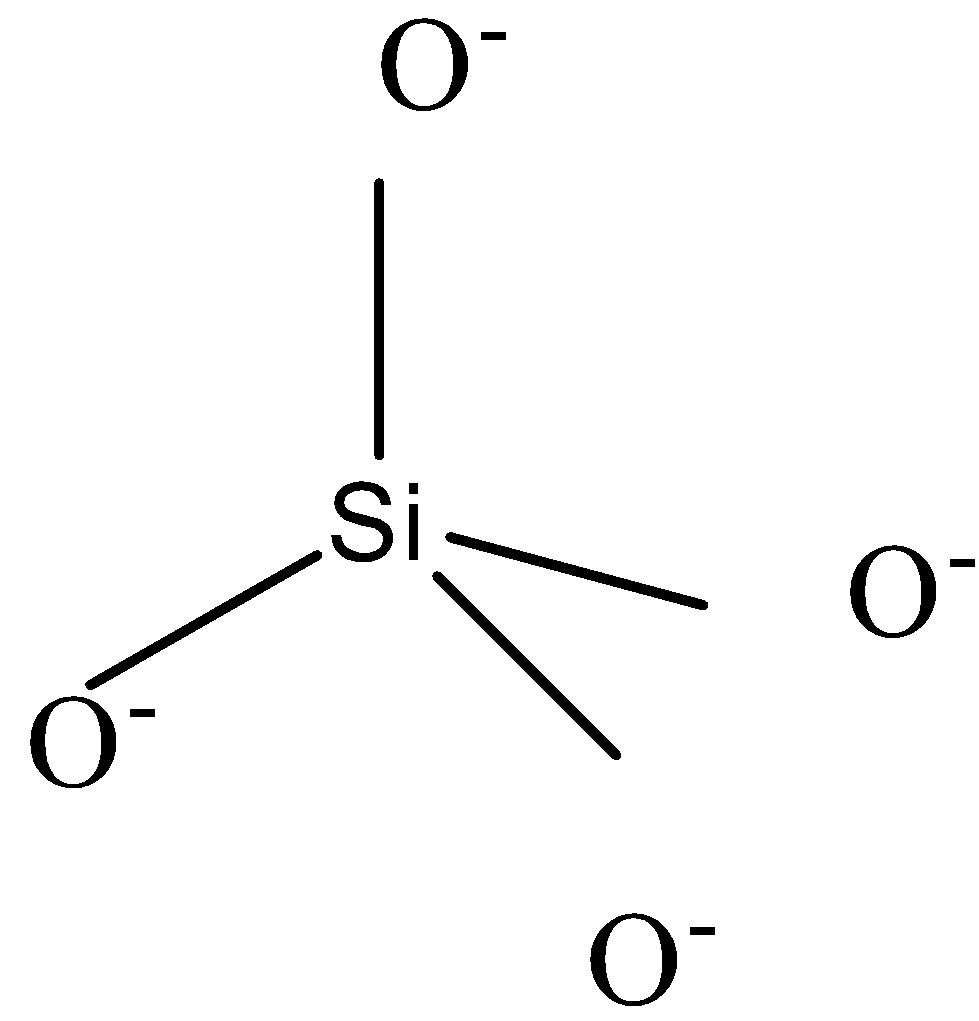
-The other compound has one oxygen atom less. So to complete the octet of silicon, we need to connect 2 bonds of silicon with those of any 1 oxygen atom. The other 2 oxygen atoms will be drawn just as those in ${{\left[ Si{{O}_{4}} \right]}^{4-}}$. Thus the charge of a compound with 3 oxygen atoms will be -2. The shape can be shown as
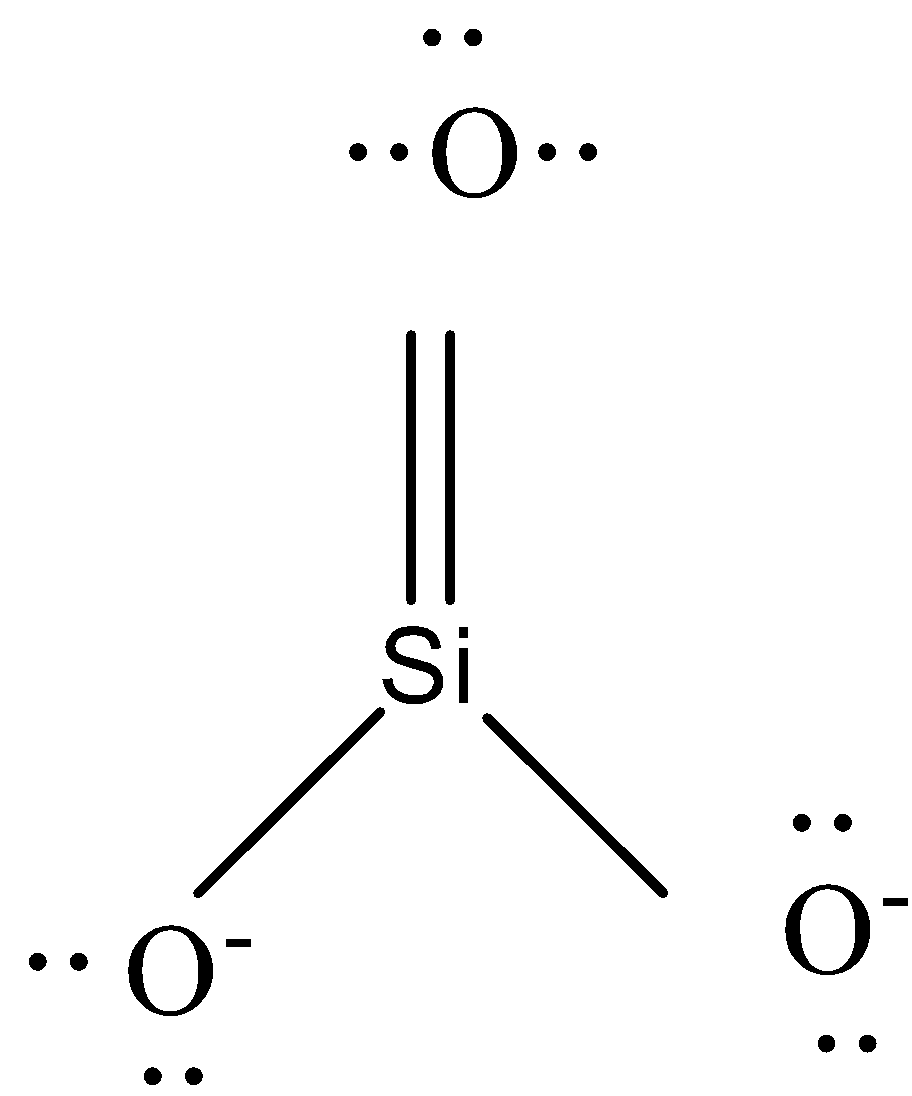
So it is a trigonal planar as there are no lone pair of electrons in silicon.
Thus the correct option is A. Two-dimensional sheet structure.
Note: There are 2 varieties of sheet structures that can be drawn for silicates. One is based on ${{\left[ Si{{O}_{4}} \right]}^{4-}}$tetrahedron shown above. Another is graphene-like sheets. Both of them have hexagonal crystal symmetry. They are used as dielectrics in the electronics industry.
Hint: VSEPR theory states that the electron pairs repel each other, whether they are in the form of lone pair or bond pair. This is done in order to minimise the repulsion, thus increasing the stability of the compound.
Complete step by step solution:
-To draw the structure of any molecule, we must know the group of the atoms present in the molecule. The groups tell us about the valence electrons of the atom. This in turn gives us the number of bonds needed to be connected to the atom to complete its octet. If there are less number of bonds for an atom, then we represent it with a negative sign.
-To talk about ${{\left[ Si{{O}_{4}} \right]}^{4-}}$
Here we see that silicon is the centrala tom and it lies in group 4 while oxygen in group 16. So, silicon should have 4 bonds while oxygen should have 2 bonds. Now as there are 4 oxygen atoms, we link those to silicon to complete the octet of silicon. Since there are no more atoms, the octet of oxygen atoms is not complete and so they are represented by a negative sign. Thus, the total charge present on the molecule is -4.
-Since there are 4 bonds which are all identical, the shape of this compound will be tetrahedral and can be shown as

-The other compound has one oxygen atom less. So to complete the octet of silicon, we need to connect 2 bonds of silicon with those of any 1 oxygen atom. The other 2 oxygen atoms will be drawn just as those in ${{\left[ Si{{O}_{4}} \right]}^{4-}}$. Thus the charge of a compound with 3 oxygen atoms will be -2. The shape can be shown as

So it is a trigonal planar as there are no lone pair of electrons in silicon.
Thus the correct option is A. Two-dimensional sheet structure.
Note: There are 2 varieties of sheet structures that can be drawn for silicates. One is based on ${{\left[ Si{{O}_{4}} \right]}^{4-}}$tetrahedron shown above. Another is graphene-like sheets. Both of them have hexagonal crystal symmetry. They are used as dielectrics in the electronics industry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




