
What is the Lewis Dot Diagram for Platinum?
Answer
524.4k+ views
Hint :Lewis dots are the structure which helps one to identify the number of electrons present in the Valence shell of any element. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
Complete Step By Step Answer:
To Assign dot structure, we will consider only the valence shell electrons of elements.
\[\]
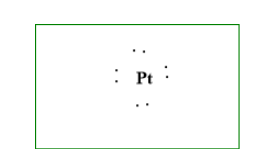
\[1.\]The first step is to write the chemical symbol. Here it is\[Pt\].
The atomic number of platinum\[(Pt)\]is\[78\]. In the periodic table, this falls in the\[{10^{th}}\] and \[{6^{th}}\]period.
Platinum has electronic configuration of: \[{}^{78}Pt = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}4{p^6}5{s^2}4{d^{10}}5{p^6}6{s^2}4{f^{14}}5{d^8}\]
Or the abbreviated configuration is: \[Pt = [{}^{54}Xe]6{s^2}4{f^{14}}5{d^8}\]
It is clear that platinum has only \[8\] electrons in the last shell which is the valence shell.
\[2.\]Second step is to find the valence electron, that is\[8\].
\[3.\] Third is the last step, is to draw the structure.
Additional Information:
The columns, or groups, table are in the periodic table used to determine the number of valence electrons for each element. Each column of the table contains elements that have the same number of columns from the left edge of the table tells us the exact number of valence electrons for that element.
Note :
The noble gas is chemically stable and has a full valence level of electrons. Other elements react in order to have the full valence level of electrons. Lewis symbols represent the valence electrons as dots surrounding the elements symbol for the atom. Once you can draw a Lewis symbol for an atom, you can use this knowledge to create dot structure of molecules.
Complete Step By Step Answer:
To Assign dot structure, we will consider only the valence shell electrons of elements.
\[\]
\[1.\]The first step is to write the chemical symbol. Here it is\[Pt\].
The atomic number of platinum\[(Pt)\]is\[78\]. In the periodic table, this falls in the\[{10^{th}}\] and \[{6^{th}}\]period.
Platinum has electronic configuration of: \[{}^{78}Pt = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}4{p^6}5{s^2}4{d^{10}}5{p^6}6{s^2}4{f^{14}}5{d^8}\]
Or the abbreviated configuration is: \[Pt = [{}^{54}Xe]6{s^2}4{f^{14}}5{d^8}\]
It is clear that platinum has only \[8\] electrons in the last shell which is the valence shell.
\[2.\]Second step is to find the valence electron, that is\[8\].
\[3.\] Third is the last step, is to draw the structure.

Additional Information:
The columns, or groups, table are in the periodic table used to determine the number of valence electrons for each element. Each column of the table contains elements that have the same number of columns from the left edge of the table tells us the exact number of valence electrons for that element.
Note :
The noble gas is chemically stable and has a full valence level of electrons. Other elements react in order to have the full valence level of electrons. Lewis symbols represent the valence electrons as dots surrounding the elements symbol for the atom. Once you can draw a Lewis symbol for an atom, you can use this knowledge to create dot structure of molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




