
What is the Lewis dot structure for $ Br{O_2}^ - $ and what are the formal charges on each atom?
Answer
516.3k+ views
Hint: Polyatomic ions are charged complexes made up of one or more elements, with one or more of the atoms being charged. The formal charge of a polyatomic ion is equal to the number of the charges of all the ions present in the compound. Some of the polyatomic ions are formed when a positively charged ion leaves the compound while the electron remains within.
Complete answer:
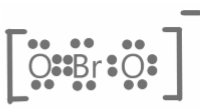
Bromite ion is a polyatomic ion made up of one bromine atom and two oxygen atoms, one of which has a double bond with the bromine atom and the other having a single bond with the bromine atom. Since the oxygen atom with the single bond has an extra valence electron, the product has a negative formal charge. The ion bromide is formed when the compound bromous acid is deprotonated, resulting in a single bond between bromine and oxygen, as well as a single bond between bromine and a hydrogen atom. However, the acid produces a hydrogen ion, which leaves the electron in the oxygen atom's valence shell.
The valence shell of bromine has seven electrons, while the valence shell of oxygen has six. If one of the oxygen atoms gains an extra valence electron, the total number of electrons in the valence shell increases to seven. That is, for the compound the total number of valence electrons is 7 + 7 + 6 = 20.
A formal charge (FC) is the charge allocated to an atom in a molecule based on the assumption that electrons in all chemical bonds are exchanged equally among atoms, regardless of relative electronegativity. When choosing the best Lewis structure (or prevailing resonance structure) for a molecule, it is important to keep the formal charge on each of the atoms as low as possible.
$ FC = V - N - \dfrac{B}{2} $
where V is the total number of valence electrons shared in bonds with other atoms in the molecule; N is the number of nonbonding valence electrons on this atom in the molecule; and B is the total number of electrons shared in bonds with other atoms in the molecule.
Note:
This disparity in practise stems from the relatively straightforward assignment of bond order, valence electron count, and hence formal charge for compounds containing only main-group elements (though oligomeric compounds like organolithium reagents and enolates seem to be depicted in an oversimplified and idealised manner), whereas there are genuine uncertainties, ambiguities, and outright disagrity for compounds containing only minor-group elements.
Complete answer:
Bromite ion is a polyatomic ion made up of one bromine atom and two oxygen atoms, one of which has a double bond with the bromine atom and the other having a single bond with the bromine atom. Since the oxygen atom with the single bond has an extra valence electron, the product has a negative formal charge. The ion bromide is formed when the compound bromous acid is deprotonated, resulting in a single bond between bromine and oxygen, as well as a single bond between bromine and a hydrogen atom. However, the acid produces a hydrogen ion, which leaves the electron in the oxygen atom's valence shell.
The valence shell of bromine has seven electrons, while the valence shell of oxygen has six. If one of the oxygen atoms gains an extra valence electron, the total number of electrons in the valence shell increases to seven. That is, for the compound the total number of valence electrons is 7 + 7 + 6 = 20.

A formal charge (FC) is the charge allocated to an atom in a molecule based on the assumption that electrons in all chemical bonds are exchanged equally among atoms, regardless of relative electronegativity. When choosing the best Lewis structure (or prevailing resonance structure) for a molecule, it is important to keep the formal charge on each of the atoms as low as possible.
$ FC = V - N - \dfrac{B}{2} $
where V is the total number of valence electrons shared in bonds with other atoms in the molecule; N is the number of nonbonding valence electrons on this atom in the molecule; and B is the total number of electrons shared in bonds with other atoms in the molecule.
| Br | O(double bonded) | O(single bonded) | |
| V | 7 | 6 | 6 |
| N | 4 | 4 | 6 |
| B | 6 | 4 | 2 |
| $ \dfrac{B}{2} $ | 3 | 2 | 1 |
| F | 0 | 0 | -1 |
Note:
This disparity in practise stems from the relatively straightforward assignment of bond order, valence electron count, and hence formal charge for compounds containing only main-group elements (though oligomeric compounds like organolithium reagents and enolates seem to be depicted in an oversimplified and idealised manner), whereas there are genuine uncertainties, ambiguities, and outright disagrity for compounds containing only minor-group elements.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




