
Lewis structure for :
$ ClO_3^- $ (chlorate ion)
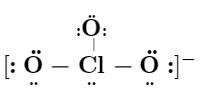
If the above Lewis structure is correct enter $ 1 $ else enter $ 0 $.
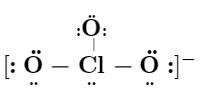
Answer
518.7k+ views
Hint :For solving this problem, you should have the knowledge about polyatomic ion and oxidation number.Polyatomic ion is an ion containing more than one atom. Oxidation number is the number of electrons lost or gained by an element during a reaction.
Complete Step By Step Answer:
Step-1 :
To solve the given problem, let’s determine the valence electron of all the atoms present in the compound.
Step-2 :
Here, we have given chocolate ions. So, know that the total charge on the ion should be $ ClO_3^- $ . So, we know that the total charge on the ion should be $ -1 $ . We should first find the total valence electron. Here, chlorine has $ 1 $ atom and contains $ 7 $ electrons and oxygen atoms contain $ 18 $ valence electrons.
So, total valence electron is :
$ 7+18-(-1)=26 $
Step-3 :
Next we should find total electron pairs which is a sum of bond pairs and lone pairs. The total electron pairs can be calculated by dividing total valence electrons with $ 2 $ . Here, dividing $ 26 $ by $ 2 $ , we get total electron pairs as $ 13 $ .
Step-4 :
Here, chlorine is used as the central atom and oxygen as the surrounding atom. Here, maximum valency of chlorine is $ 7 $ while that of oxygen is $ 2 $ . So, oxygen can maximum from $ 2 $ bonds while chlorine can form $ 7 $ bonds. The charge on $ 1 $ oxygen is $ -1 $ .
Note :
While determining the Lewis structure of a compound, the valence electron should be remembered and determined correctly so as to avoid any mistakes and if possible the Lewis structures of some basic compounds can be learned to simplify the process.
Complete Step By Step Answer:
Step-1 :
To solve the given problem, let’s determine the valence electron of all the atoms present in the compound.
Step-2 :
Here, we have given chocolate ions. So, know that the total charge on the ion should be $ ClO_3^- $ . So, we know that the total charge on the ion should be $ -1 $ . We should first find the total valence electron. Here, chlorine has $ 1 $ atom and contains $ 7 $ electrons and oxygen atoms contain $ 18 $ valence electrons.
So, total valence electron is :
$ 7+18-(-1)=26 $
Step-3 :
Next we should find total electron pairs which is a sum of bond pairs and lone pairs. The total electron pairs can be calculated by dividing total valence electrons with $ 2 $ . Here, dividing $ 26 $ by $ 2 $ , we get total electron pairs as $ 13 $ .
Step-4 :
Here, chlorine is used as the central atom and oxygen as the surrounding atom. Here, maximum valency of chlorine is $ 7 $ while that of oxygen is $ 2 $ . So, oxygen can maximum from $ 2 $ bonds while chlorine can form $ 7 $ bonds. The charge on $ 1 $ oxygen is $ -1 $ .
Note :
While determining the Lewis structure of a compound, the valence electron should be remembered and determined correctly so as to avoid any mistakes and if possible the Lewis structures of some basic compounds can be learned to simplify the process.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




