
What is the Lewis structure of nitrosyl chloride, $ NOCl $ ?
Answer
491.7k+ views
Hint: Chemical elements are the purest form of atoms, and atoms of an element combine with each other and form molecules. Nitrogen, oxygen and chlorine combine to form a compound known as nitrosyl chloride. The Lewis structure of nitrosyl chloride can be explained by the valence electrons on all the atoms in the compound.
Complete answer:
Lewis structure represents the bonding pair of electrons and lone pair of electrons. Out of the valence electrons on each atom in the molecule some electrons exist as lone pairs and some will exist as bond pairs of electrons.
Nitrogen is an element with atomic number $ 7 $ and the valence electrons on nitrogen atom are $ 5 $
Oxygen is an element with atomic number $ 8 $ and the valence electrons on nitrogen atom are $ 6 $
Chlorine is an element with atomic number $ 8 $ and the valence electrons on nitrogen atom are $ 7 $
The total valence electrons on all the atoms in nitrosyl chloride are $ 7 + 6 + 5 = 18 $
Out of these $ 18 $ valence electrons, six electrons are existing as three bonds, out of these three bonds two bonds are formed between nitrogen and oxygen, one bond was formed between nitrogen and chlorine.
The Lewis structure of nitrosyl chloride is
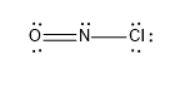
Note:
In the Lewis structure of any molecule, the atom with lowest electronegativity value occupies a central position. The given molecule is nitrosyl chloride, in which the central metal atom is nitrogen as it has less electronegativity value when compared to the atoms of oxygen and chlorine.
Complete answer:
Lewis structure represents the bonding pair of electrons and lone pair of electrons. Out of the valence electrons on each atom in the molecule some electrons exist as lone pairs and some will exist as bond pairs of electrons.
Nitrogen is an element with atomic number $ 7 $ and the valence electrons on nitrogen atom are $ 5 $
Oxygen is an element with atomic number $ 8 $ and the valence electrons on nitrogen atom are $ 6 $
Chlorine is an element with atomic number $ 8 $ and the valence electrons on nitrogen atom are $ 7 $
The total valence electrons on all the atoms in nitrosyl chloride are $ 7 + 6 + 5 = 18 $
Out of these $ 18 $ valence electrons, six electrons are existing as three bonds, out of these three bonds two bonds are formed between nitrogen and oxygen, one bond was formed between nitrogen and chlorine.
The Lewis structure of nitrosyl chloride is
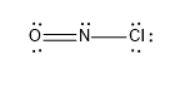
Note:
In the Lewis structure of any molecule, the atom with lowest electronegativity value occupies a central position. The given molecule is nitrosyl chloride, in which the central metal atom is nitrogen as it has less electronegativity value when compared to the atoms of oxygen and chlorine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




