
Line spectrum is characteristic of:
a.) Molecules
b.) Atoms
c.) Radicals
d.) None of the above
Answer
600.3k+ views
Hint: "Line spectrum" generally implies that we are talking about lines with wavelengths that fall into the range of the visible spectrum. Now you can easily answer this one.
Complete step by step answer:
The line spectrum is the result of the interaction between a quantum system and a single photon. When a photon has about the right amount of energy to allow a change in the energy state of the system, the photon is absorbed.
The photon will be spontaneously re-emitted, either in the same frequency as the original or in a cascade, where the sum of the energies of the photons emitted will be equal to the energy of the one absorbed.
When atoms are excited they emit light of certain wavelengths that correspond to different colors and the emitted light is observed as a series of colored lines with dark spaces in between. This series of colored lines is called a line or atomic spectra.
Each element produces a unique set of spectral lines and since no two elements emit the same spectral lines, elements can be identified by their line spectrum.
Here we can see electron transitions for hydrogen atom -
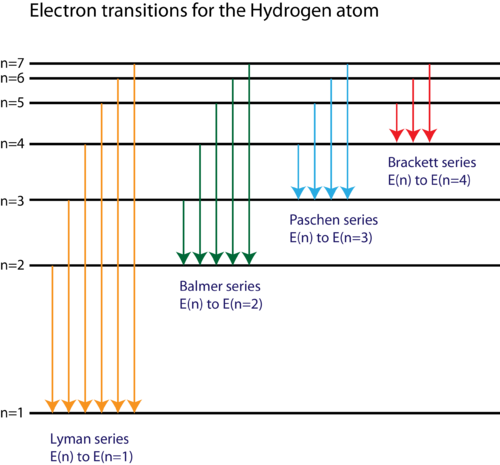
Therefore, we can conclude that the correct answer to this question is option B.
Note: We should know that spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected "fingerprints" of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.
Complete step by step answer:
The line spectrum is the result of the interaction between a quantum system and a single photon. When a photon has about the right amount of energy to allow a change in the energy state of the system, the photon is absorbed.
The photon will be spontaneously re-emitted, either in the same frequency as the original or in a cascade, where the sum of the energies of the photons emitted will be equal to the energy of the one absorbed.
When atoms are excited they emit light of certain wavelengths that correspond to different colors and the emitted light is observed as a series of colored lines with dark spaces in between. This series of colored lines is called a line or atomic spectra.
Each element produces a unique set of spectral lines and since no two elements emit the same spectral lines, elements can be identified by their line spectrum.
Here we can see electron transitions for hydrogen atom -

Therefore, we can conclude that the correct answer to this question is option B.
Note: We should know that spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected "fingerprints" of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




