
When liquid ‘A’ is treated with a freshly prepared ammoniacal silver nitrate solution, it gives a bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogensulphite. Liquid ‘B’ also forms a white crystalline solid with sodium hydrogensulphite but it does not test with ammoniacal silver nitrate. Which of the two liquids is Aldehyde? Write the chemical equations of these reactions also.
Answer
510.3k+ views
Hint: We are given two liquids and we are doing some tests to identify those liquids. First test involves treatment with a freshly prepared ammoniacal nitrate solution. It is also known as Tollen’s Reagent test or silver mirror test; it is used to detect the Aldehydes and Ketones. Second test involves treatment with sodium hydrogen sulphite. This test is also used to detect ketones and aldehydes.
Complete answer:
Liquid ‘A’ gives both a test and forms a silver mirror when treated with freshly prepared ammonium silver nitrate solution and it forms white crystalline solid with sodium hydrogensulphite. Now liquid ‘A’ must be an aldehyde because ketones does not give a silver mirror test. let’s see the reactions
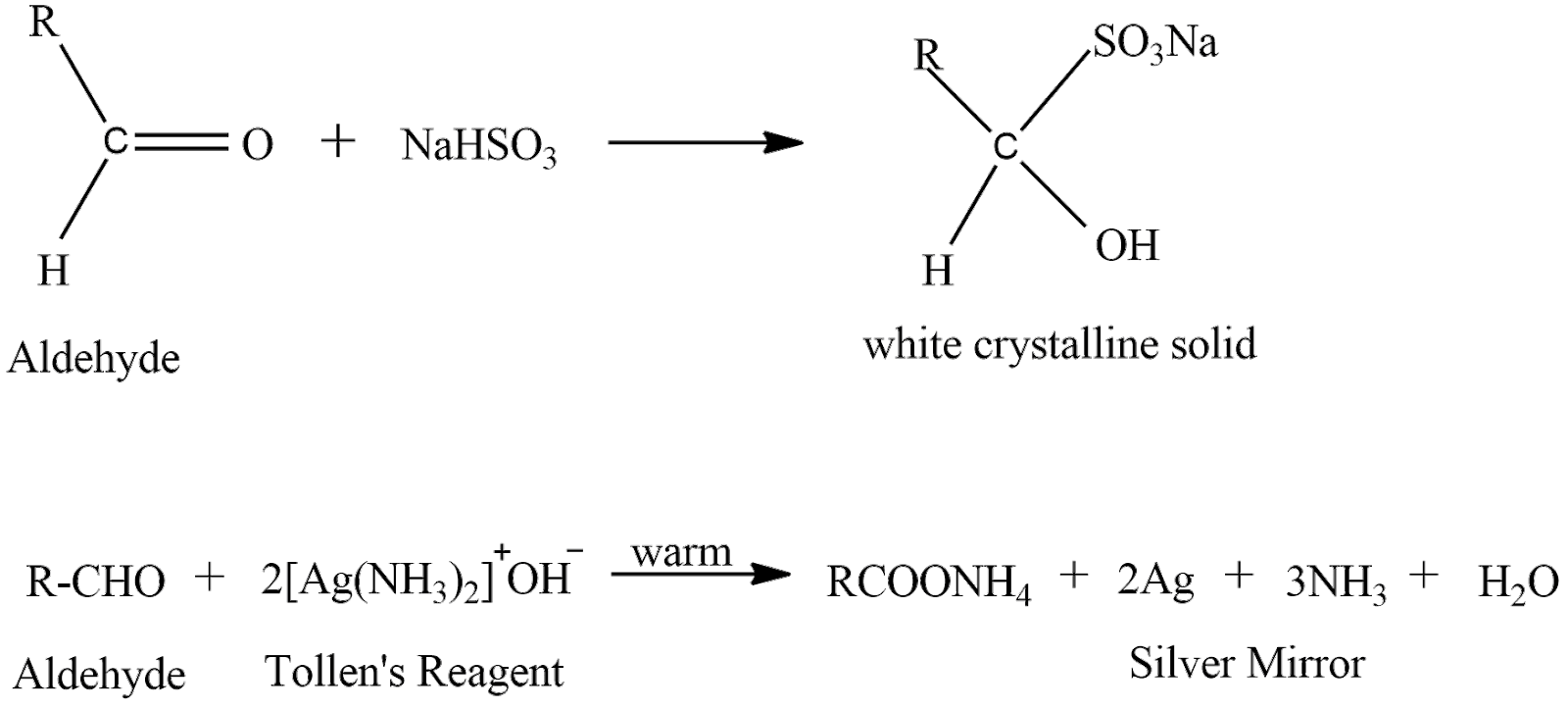
In the first reaction aldehyde reacts with sodium hydrogen sulphite to give a complex product which is a white colored crystalline solid. In the second reaction aldehyde reacts with ammoniacal silver nitrate solution to give a silver mirror.
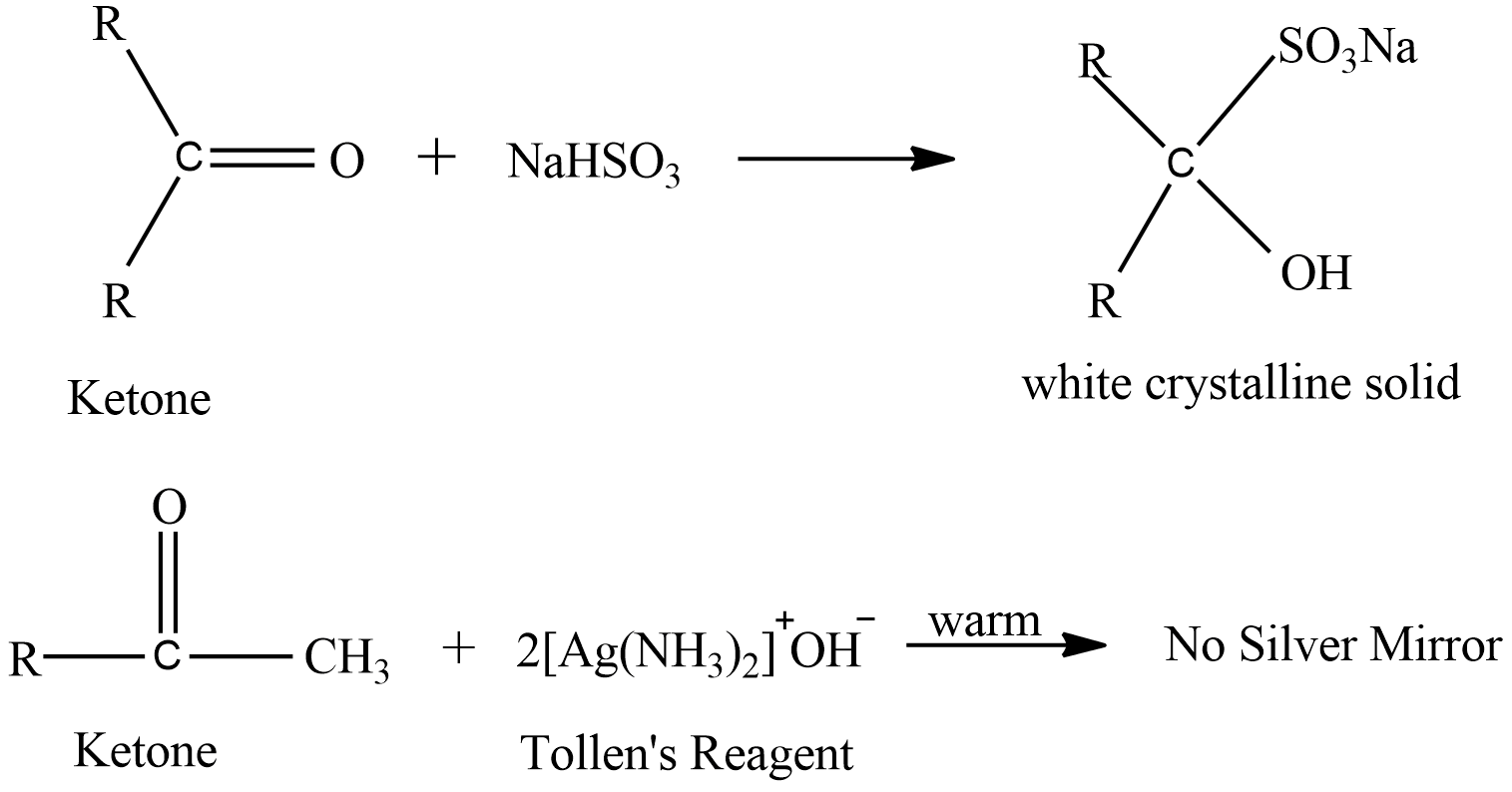
Liquid ‘B’ forms white crystalline solid when treated with sodium hydrogen sulphite but does not give silver mirror test, this signifies that liquid ‘B’ must be a ketone because ketone does not give silver mirror test. Here are the reactions
Note:
In above mentioned reactions R is any alkyl group. Sodium hydrogen sulphite is complex with both aldehyde and ketones. carefully observe the reaction to understand the process of formation of product
In the first reaction ketone reacts with sodium hydrogen sulphite to give a complex white product but it does not give silver mirror on reaction with Tollen’s reagent.
So, it is confirmed that liquid ‘A’ is aldehyde and liquid ‘B’ is ketone
Complete answer:
Liquid ‘A’ gives both a test and forms a silver mirror when treated with freshly prepared ammonium silver nitrate solution and it forms white crystalline solid with sodium hydrogensulphite. Now liquid ‘A’ must be an aldehyde because ketones does not give a silver mirror test. let’s see the reactions

In the first reaction aldehyde reacts with sodium hydrogen sulphite to give a complex product which is a white colored crystalline solid. In the second reaction aldehyde reacts with ammoniacal silver nitrate solution to give a silver mirror.
Liquid ‘B’ forms white crystalline solid when treated with sodium hydrogen sulphite but does not give silver mirror test, this signifies that liquid ‘B’ must be a ketone because ketone does not give silver mirror test. Here are the reactions

Note:
In above mentioned reactions R is any alkyl group. Sodium hydrogen sulphite is complex with both aldehyde and ketones. carefully observe the reaction to understand the process of formation of product
In the first reaction ketone reacts with sodium hydrogen sulphite to give a complex white product but it does not give silver mirror on reaction with Tollen’s reagent.
So, it is confirmed that liquid ‘A’ is aldehyde and liquid ‘B’ is ketone
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




