
How to locate the position of elements with atomic number 120 which is not yet discovered?
Answer
552.9k+ views
Hint: To locate the chemical element with atomic number of 120, the knowledge of periodic table is used. The filling of atomic orbital concept is used to know the electronic configuration of the chemical element.
Complete step by step answer:
The periodic table arranges the chemical element according to their properties. The number of elements is represented by their number of protons. In the periodic table seven rows of table are present known as periods which hold metals on the left side and nonmetals on the right side. The columns are known as groups which contain elements with similar chemical behavior. The six groups contain an assigned number of elements which are group 17 containing halogens and group 18 containing noble gases.
There are a total 118 chemical elements present in the periodic table. The last chemical element present in the periodic table is oganesson which is denoted by the chemical symbol Og. The atomic number is 118. It is placed in the lower right hand corner of the periodic table at the end of period 7 and bottom of group 18. The oganesson is the last element in the p-block element so its electronic configuration ends with $7{p^6}$
Filling of the atomic orbital is shown below.
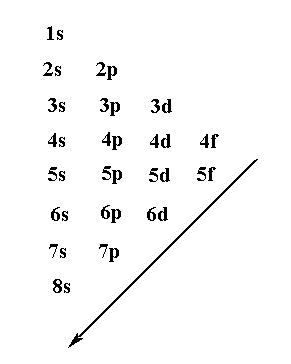
The net electron will be filled in the 8s orbital which can hold maximum 2 electrons. So the last electronic configuration of element with 120 atomic number will be $8{s^2}$ which will be present under radium with atomic number 88.
Note:
The radium is the radioactive metal. It is the heaviest alkaline earth metal so the element with atomic number 120 will also be alkaline earth metal.
Complete step by step answer:
The periodic table arranges the chemical element according to their properties. The number of elements is represented by their number of protons. In the periodic table seven rows of table are present known as periods which hold metals on the left side and nonmetals on the right side. The columns are known as groups which contain elements with similar chemical behavior. The six groups contain an assigned number of elements which are group 17 containing halogens and group 18 containing noble gases.
There are a total 118 chemical elements present in the periodic table. The last chemical element present in the periodic table is oganesson which is denoted by the chemical symbol Og. The atomic number is 118. It is placed in the lower right hand corner of the periodic table at the end of period 7 and bottom of group 18. The oganesson is the last element in the p-block element so its electronic configuration ends with $7{p^6}$
Filling of the atomic orbital is shown below.

The net electron will be filled in the 8s orbital which can hold maximum 2 electrons. So the last electronic configuration of element with 120 atomic number will be $8{s^2}$ which will be present under radium with atomic number 88.
Note:
The radium is the radioactive metal. It is the heaviest alkaline earth metal so the element with atomic number 120 will also be alkaline earth metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




