
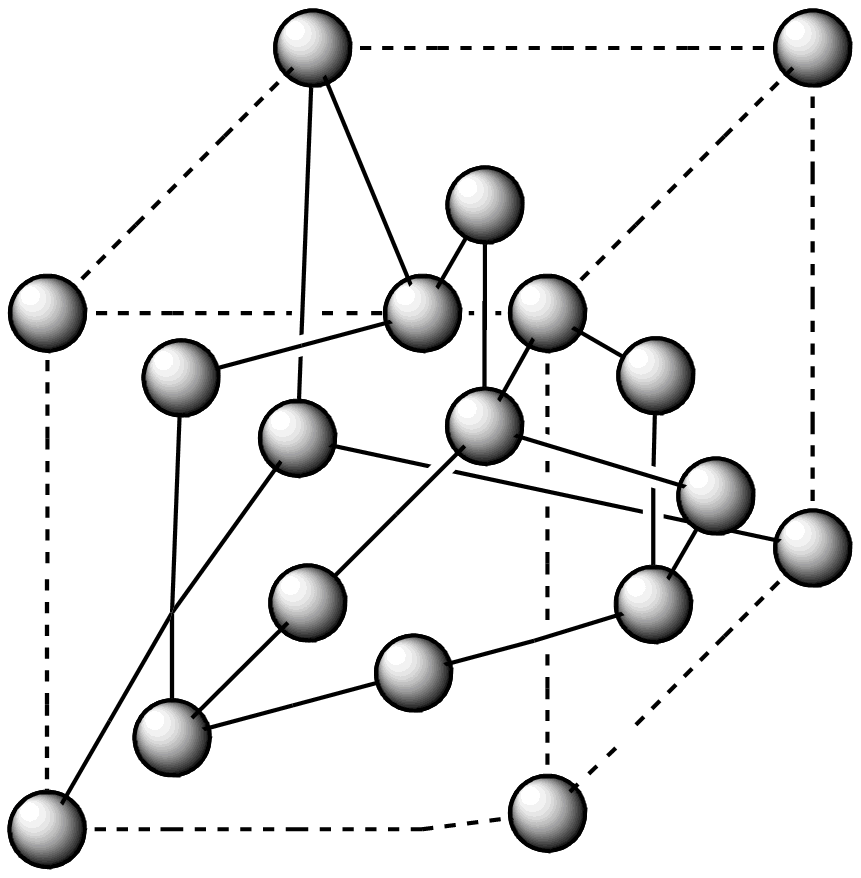
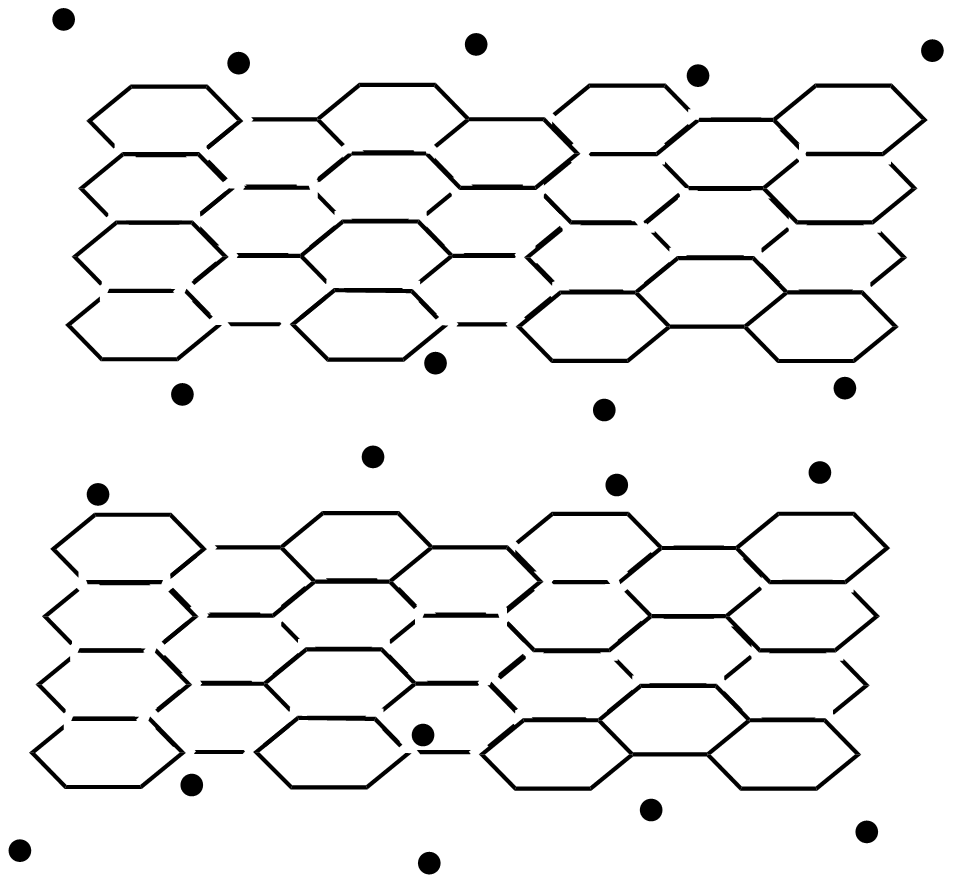
Look at the diagram and answer the following questions:
i. What type of structures do diamonds and graphite have?
ii. Why are diamonds used in cutting tools?
iii. Why is graphite used in electrical circuits?
iv. Name the force that accounts for the softness of graphite.
v. Name the precious diamond you know and give its weight in grams.


Answer
510.6k+ views
Hint: We will see each part one by one and try to solve each part very carefully. In this question structures are given of diamond and graphite. Both are the allotropes of carbon, by studying them we can know about their physical properties and predict which compound will be suited for given conditions.
Complete answer:
Diamond has a very big structure in which each carbon atom is bonded to four other carbon atoms by covalent bonds in a regular lattice arrangement which contains no free electrons. Graphite structure is also very large, each carbon atom is bonded to three other carbon atom atoms by covalent bonds the carbon atoms form layers from hexagonal arrangements of atom these layers have weak forces between them also known as van der waal forces each carbon atom in graphite contains one free electron which becomes delocalized.
Diamond is the hardest naturally occurring substance on earth. Because of its structure the tools made from diamond have high heat resistance to abrasive wear. It has high thermal stability and is chemical inert in nature that is why most cutting tools are made using diamond.
Graphite has a layered structure which allows it to have free electrons which helps it pass the electricity. free electrons make graphite a good conductor of electricity like metal that is why it is used in electrical circuits.
The graphite has a layered structure and these layers are connected to each with weak forces known as van der waal forces; these forces are responsible for the softness of graphite.
Originating from India, Kohinoor is a very famous diamond weighing 21.6 grams and 105 carats.
Note:
Both Carbon and Graphite are allotropes of carbon. Allotropes are defined as the ability of a chemical element to exist in two or more forms maintaining the same physical state and chemical properties but different physical properties like hardness, ductility, toughness, etc.
Complete answer:
Diamond has a very big structure in which each carbon atom is bonded to four other carbon atoms by covalent bonds in a regular lattice arrangement which contains no free electrons. Graphite structure is also very large, each carbon atom is bonded to three other carbon atom atoms by covalent bonds the carbon atoms form layers from hexagonal arrangements of atom these layers have weak forces between them also known as van der waal forces each carbon atom in graphite contains one free electron which becomes delocalized.
Diamond is the hardest naturally occurring substance on earth. Because of its structure the tools made from diamond have high heat resistance to abrasive wear. It has high thermal stability and is chemical inert in nature that is why most cutting tools are made using diamond.
Graphite has a layered structure which allows it to have free electrons which helps it pass the electricity. free electrons make graphite a good conductor of electricity like metal that is why it is used in electrical circuits.
The graphite has a layered structure and these layers are connected to each with weak forces known as van der waal forces; these forces are responsible for the softness of graphite.
Originating from India, Kohinoor is a very famous diamond weighing 21.6 grams and 105 carats.
Note:
Both Carbon and Graphite are allotropes of carbon. Allotropes are defined as the ability of a chemical element to exist in two or more forms maintaining the same physical state and chemical properties but different physical properties like hardness, ductility, toughness, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




