
Make the structure of neopentane, isohexane, butanoic acid and propanone along with their names.
Answer
512.1k+ views
Hint: It is important to sketch the structural formulas for organic compounds because in most of the cases, a molecular formula does not uniquely represent a single organic compound. Different compounds might have the same molecular formula but differ in structural formulas are known as isomers. Hence, sketching the structures may help in easy understanding the chemical and physical properties of each compound.
Complete answer:
When drawing the molecular structures of neutral organic compounds, the following points must be strictly followed.
1.As the valency of a carbon atom is four, so each carbon atom must consist of four bonds in the structure.
2.The valency of a nitrogen atom is three, so each nitrogen atom must consist of three bonds within its structural formula.
3.The valency of the oxygen atom is two, so each oxygen atom must consist of two bonds in the structural formula.
4.The valency of a hydrogen atom is one i.e., it must consist of only one bond within the structural formula of the organic compound.
Hence, the names and the condensed structural formula of the given organic compounds are as follows:
1. Neopentane:
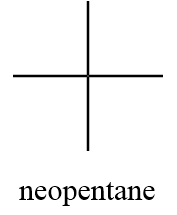
IUPAC name: 2,2-dimethylpropane
2. isohexene:
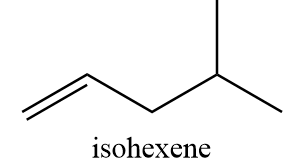
IUPAC name: 4-methylpent-1-ene
3. Butanoic acid:
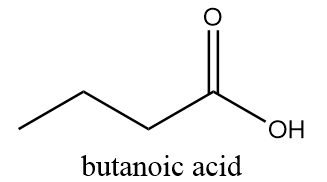
IUPAC name: Butanoic acid
4. Propanone:
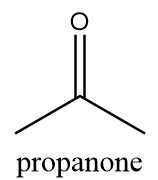
IUPAC name: propan-2-one
Hence C is the correct option.
Note:
It is important to note that an organic compound can have two names i.e., IUPAC name which is the name which follows an internationally accepted standard rules by international union of pure and applied chemistry whereas the common name are the names which do not have any common rules. As the IUPAC names are difficult to memorize, so generally common names are used to identify a compound.
Complete answer:
When drawing the molecular structures of neutral organic compounds, the following points must be strictly followed.
1.As the valency of a carbon atom is four, so each carbon atom must consist of four bonds in the structure.
2.The valency of a nitrogen atom is three, so each nitrogen atom must consist of three bonds within its structural formula.
3.The valency of the oxygen atom is two, so each oxygen atom must consist of two bonds in the structural formula.
4.The valency of a hydrogen atom is one i.e., it must consist of only one bond within the structural formula of the organic compound.
Hence, the names and the condensed structural formula of the given organic compounds are as follows:
1. Neopentane:

IUPAC name: 2,2-dimethylpropane
2. isohexene:

IUPAC name: 4-methylpent-1-ene
3. Butanoic acid:

IUPAC name: Butanoic acid
4. Propanone:

IUPAC name: propan-2-one
Hence C is the correct option.
Note:
It is important to note that an organic compound can have two names i.e., IUPAC name which is the name which follows an internationally accepted standard rules by international union of pure and applied chemistry whereas the common name are the names which do not have any common rules. As the IUPAC names are difficult to memorize, so generally common names are used to identify a compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




