
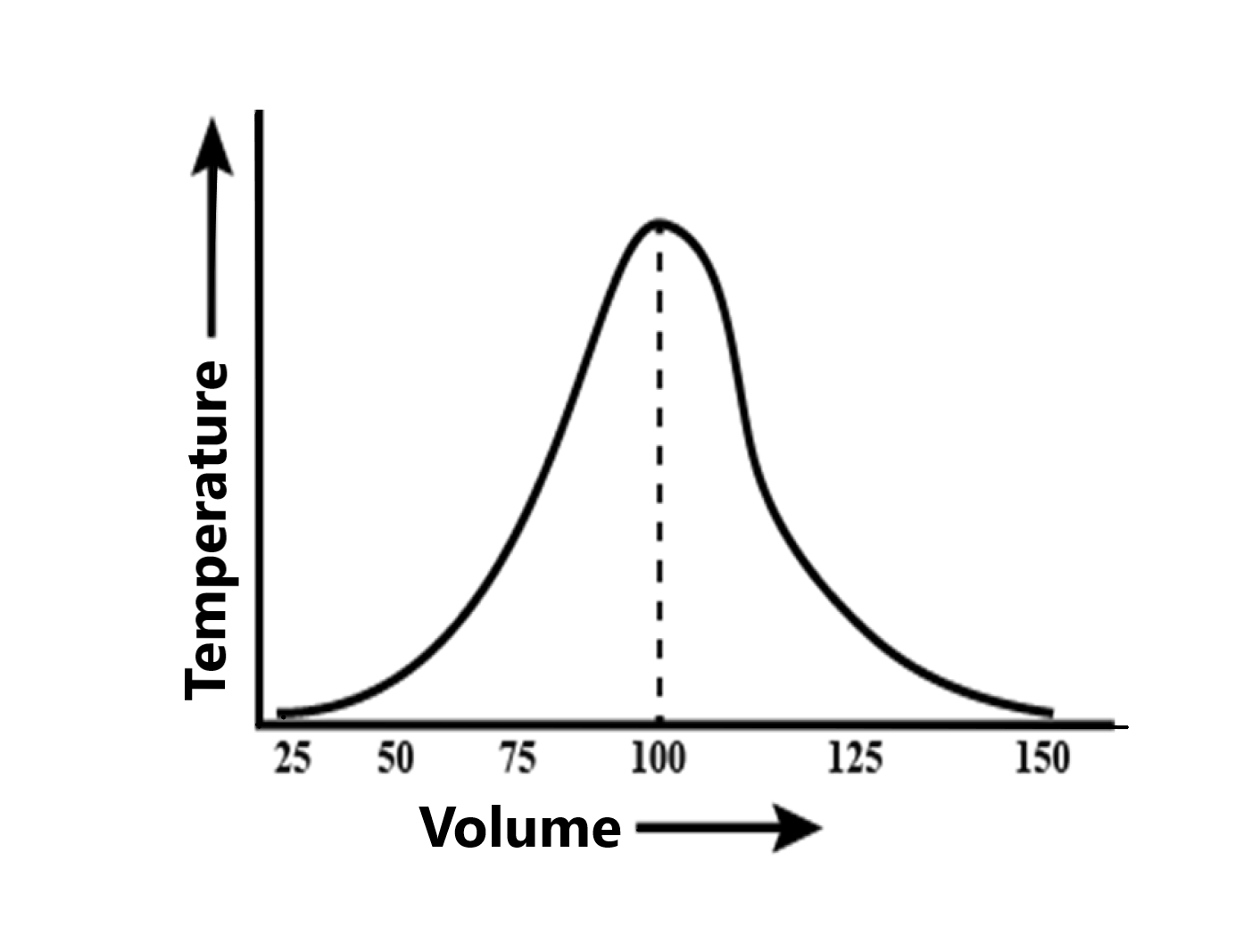
Mayuri was performing thermometric titration and she took 100 ml of 1 M sulphuric acid and started adding 1 M calcium hydroxide and she plotted a graph of temperature vs volume of the titrant added. In that experiment, she found that temperature was initially increasing and then it started decreasing. The maximum of the graph is obtained at 100 ml. calcium hydroxide. What will be the enthalpy change of this reaction?
(Given $\text{ }\Delta \text{H = -13}\text{.7 kcal }$ for equivalent)
A) $\text{ -17}\text{.3 kcal }$
B) $\text{ -27}\text{.4 kcal }$
C) $\text{ -1}\text{.73 kcal }$
D) $\text{ -2}\text{.74 kcal }$

Answer
578.4k+ views
Hint: The enthalpy of neutralization of one mole of a base such as $\text{ NaOH }$ ,$\text{ Ca(OH}{{\text{)}}_{\text{2}}}\text{ }$ by acid as $\text{ HCl }$ , $\text{ }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$ in dilute solution at $\text{ 2}{{\text{5}}^{\text{0}}}\text{C }$and 1 atm pressure is called the standard enthalpy of the neutralization reaction. The enthalpy change for the reaction can be studied by the thermometric titrations. It is a plot of the temperature of the solution (acid reacting with base) against the volume of base or acid added .the highest peak in the graph indicates the temperature and volume at which reaction undergoes the completion.
Complete answer:
Thermometric titration is the type of titration reaction in which the titrant is added to the titrant until the reaction is complete. The completion of the reaction is determined by the change in temperature.
Here, we have given the following data,
The volume of sulphuric acid, $\text{ }{{\text{V}}_{\text{1}}}\text{=100ml}$
The concentration of sulphuric acid,$\text{ }{{\text{M}}_{\text{1}}}\text{= 1 M }$
The concentration of calcium hydroxide, $\text{ }{{\text{M}}_{2}}\text{= 1 M }$
The enthalpy of the reaction is $\text{ }\Delta \text{H = -13}\text{.7 kcal }$
The acid reacts with the base and the volume required for the neutralization of acid was the Volume of calcium hydroxide.
This can be also determined by the plot. Here, the thermometric titration is studied by plotting a graph of temperature against the volume. On the addition of titrant to the titrand, the temperature of the solution rises from room temperature and reaches to the highest temperature.
After this, we can observe a gradual decrease in the temperature of the system.in titration reactions, the highest temperature is the temperature at which the reaction undergoes the completion. Thus, the volume corresponding to this temperature is considered as the volume of base required to completely neutralize the acid.
We have to find the enthalpy of change of the reaction.
Enthalpy of neutralization reaction of one mole of the base such as the calcium hydroxide by the acid $\text{ }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$at the $\text{ 2}{{\text{5}}^{\text{0}}}\text{C }$ and 1 atm pressure is called the enthalpy of a neutralization reaction. The reaction is as shown below,
$\text{ 2}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ + Ca(OH}{{\text{)}}_{\text{2}}}\text{ }\to \text{ 2CaS}{{\text{O}}_{\text{4}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }\Delta \text{H = -13}\text{.7 kcal }$
Considering the neutralization of strong acid by the strong base, it can be seen that the neutralization reaction is the same as it simply involves the combination of $\text{ }{{\text{H}}^{\text{+}}}\text{ }$ and $\text{ O}{{\text{H}}^{-}}\text{ }$ ions to form an unionized water molecule. Therefore, it is expected that enthalpy change of the neutralization reaction of strong base and acid and vice versa should be identical.
Therefore, here the total enthalpy change in for the reaction of 1 molar sulphuric acid with the 1 molar calcium hydroxide is equal to$\text{ }\Delta \text{H = -13}\text{.7 kcal }$.
Hence, (A) is the correct option.
Note:
Note that, here strong acid is reacting with a strong base thus the enthalpy change for the reaction is taken as the same value provided. However, if acid or alkali is weak, then the enthalpy of neutralization would be different because the reaction now involves the partial dissociation of weak acid or alkali. For example, for acetic acid, the enthalpy of neutralization by sodium hydroxide is $\text{ -55}\text{.23 k J mo}{{\text{l}}^{\text{-1}}}\text{ }$. Since the average value of the combination of $\text{ }{{\text{H}}^{\text{+}}}\text{ }$and $\text{ O}{{\text{H}}^{-}}\text{ }$ions is taken as the $\text{ -57}\text{.32 k J mo}{{\text{l}}^{\text{-1}}}\text{ }$ standard enthalpy of dissociation of acetic acid would be, $\text{ }\Delta \text{H= -55}\text{.23 k J mo}{{\text{l}}^{\text{-1}}}\text{ -( -57}\text{.32 k J mo}{{\text{l}}^{\text{-1}}}\text{ })=+2.09\text{ k J mo}{{\text{l}}^{-1}}\text{ }$.
Complete answer:
Thermometric titration is the type of titration reaction in which the titrant is added to the titrant until the reaction is complete. The completion of the reaction is determined by the change in temperature.
Here, we have given the following data,
The volume of sulphuric acid, $\text{ }{{\text{V}}_{\text{1}}}\text{=100ml}$
The concentration of sulphuric acid,$\text{ }{{\text{M}}_{\text{1}}}\text{= 1 M }$
The concentration of calcium hydroxide, $\text{ }{{\text{M}}_{2}}\text{= 1 M }$
The enthalpy of the reaction is $\text{ }\Delta \text{H = -13}\text{.7 kcal }$
The acid reacts with the base and the volume required for the neutralization of acid was the Volume of calcium hydroxide.
This can be also determined by the plot. Here, the thermometric titration is studied by plotting a graph of temperature against the volume. On the addition of titrant to the titrand, the temperature of the solution rises from room temperature and reaches to the highest temperature.
After this, we can observe a gradual decrease in the temperature of the system.in titration reactions, the highest temperature is the temperature at which the reaction undergoes the completion. Thus, the volume corresponding to this temperature is considered as the volume of base required to completely neutralize the acid.
We have to find the enthalpy of change of the reaction.
Enthalpy of neutralization reaction of one mole of the base such as the calcium hydroxide by the acid $\text{ }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ }$at the $\text{ 2}{{\text{5}}^{\text{0}}}\text{C }$ and 1 atm pressure is called the enthalpy of a neutralization reaction. The reaction is as shown below,
$\text{ 2}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ + Ca(OH}{{\text{)}}_{\text{2}}}\text{ }\to \text{ 2CaS}{{\text{O}}_{\text{4}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }\Delta \text{H = -13}\text{.7 kcal }$
Considering the neutralization of strong acid by the strong base, it can be seen that the neutralization reaction is the same as it simply involves the combination of $\text{ }{{\text{H}}^{\text{+}}}\text{ }$ and $\text{ O}{{\text{H}}^{-}}\text{ }$ ions to form an unionized water molecule. Therefore, it is expected that enthalpy change of the neutralization reaction of strong base and acid and vice versa should be identical.
Therefore, here the total enthalpy change in for the reaction of 1 molar sulphuric acid with the 1 molar calcium hydroxide is equal to$\text{ }\Delta \text{H = -13}\text{.7 kcal }$.
Hence, (A) is the correct option.
Note:
Note that, here strong acid is reacting with a strong base thus the enthalpy change for the reaction is taken as the same value provided. However, if acid or alkali is weak, then the enthalpy of neutralization would be different because the reaction now involves the partial dissociation of weak acid or alkali. For example, for acetic acid, the enthalpy of neutralization by sodium hydroxide is $\text{ -55}\text{.23 k J mo}{{\text{l}}^{\text{-1}}}\text{ }$. Since the average value of the combination of $\text{ }{{\text{H}}^{\text{+}}}\text{ }$and $\text{ O}{{\text{H}}^{-}}\text{ }$ions is taken as the $\text{ -57}\text{.32 k J mo}{{\text{l}}^{\text{-1}}}\text{ }$ standard enthalpy of dissociation of acetic acid would be, $\text{ }\Delta \text{H= -55}\text{.23 k J mo}{{\text{l}}^{\text{-1}}}\text{ -( -57}\text{.32 k J mo}{{\text{l}}^{\text{-1}}}\text{ })=+2.09\text{ k J mo}{{\text{l}}^{-1}}\text{ }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




