
What do you mean by anomalous expansion of water?
Answer
599.1k+ views
Hint: Consider the expansion and contraction of water with variation of temperature. Compare this behaviour of water with other substances. Also consider the change in density with temperature.
Complete step-by-step answer:
As the temperature decreases all the substances contract and their density increases. But this is not the case with water, water contracts as its temperature is decreased except from 4 degree centigrade to zero degree centigrade. In this temperature range, instead of contracting water expands and density becomes less and less. This abnormal property of water is known as anomalous expansion of water.
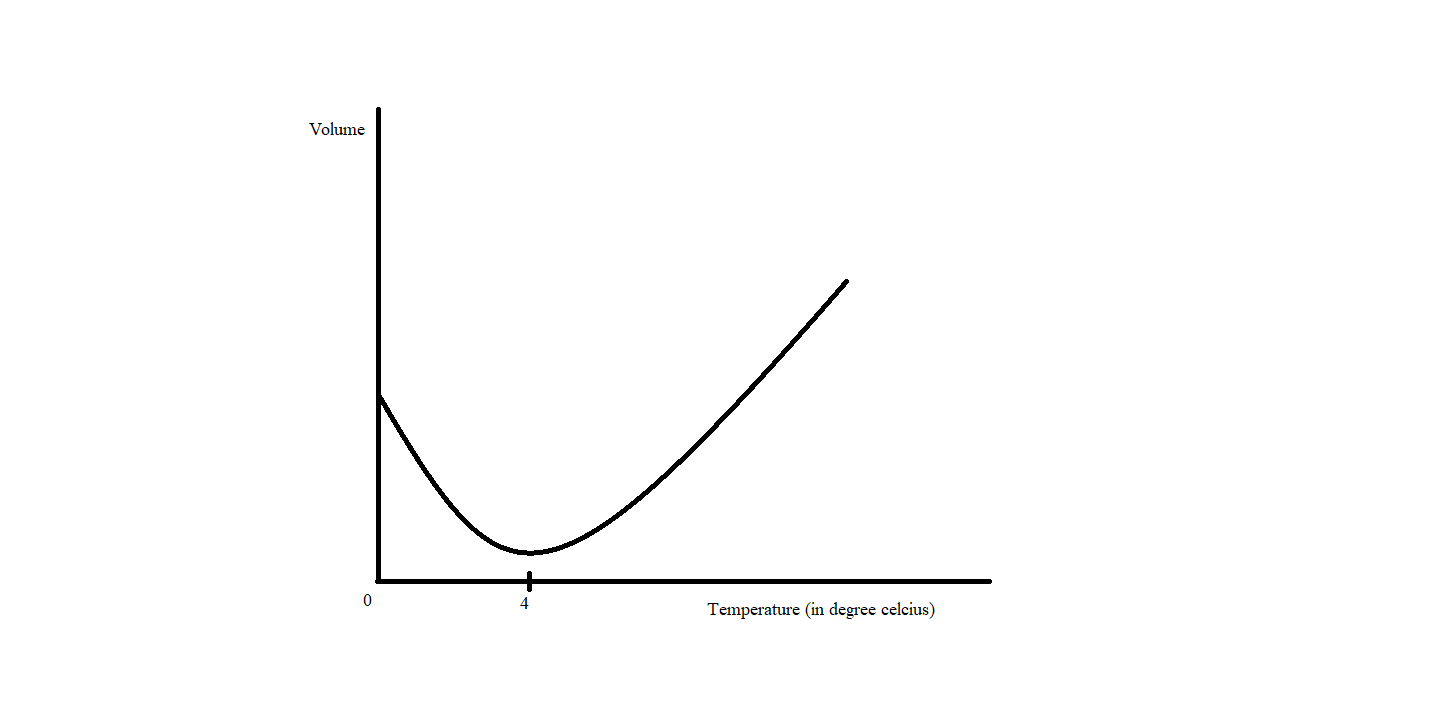
Why this happens:
Water molecule is made up of two oxygen and one hydrogen atom with a strong intermolecular force of attraction. At normal temperature water molecules move inside a container freely in random motion. On cooling as the energy of water molecules decreases and random motion of water molecules squeezes. As we decrease the temperature, water molecules come closer and closer and hydrogen bonds are formed between hydrogen atoms of one molecule and oxygen atoms of other molecules. This formation of hydrogen bonds prevents further contraction of water and density of water reaches its maximum value. This unusual property of water helps marine life to survive in polar regions where the average temperature is below zero centigrade.
Note: Because of this kind of strange behaviour ice (solid state of water) is lighter than liquid water. Cold water floats above warm water. Water bodies start freezing from top to bottom. This type of behaviour is observed only in water.
Complete step-by-step answer:
As the temperature decreases all the substances contract and their density increases. But this is not the case with water, water contracts as its temperature is decreased except from 4 degree centigrade to zero degree centigrade. In this temperature range, instead of contracting water expands and density becomes less and less. This abnormal property of water is known as anomalous expansion of water.

Why this happens:
Water molecule is made up of two oxygen and one hydrogen atom with a strong intermolecular force of attraction. At normal temperature water molecules move inside a container freely in random motion. On cooling as the energy of water molecules decreases and random motion of water molecules squeezes. As we decrease the temperature, water molecules come closer and closer and hydrogen bonds are formed between hydrogen atoms of one molecule and oxygen atoms of other molecules. This formation of hydrogen bonds prevents further contraction of water and density of water reaches its maximum value. This unusual property of water helps marine life to survive in polar regions where the average temperature is below zero centigrade.
Note: Because of this kind of strange behaviour ice (solid state of water) is lighter than liquid water. Cold water floats above warm water. Water bodies start freezing from top to bottom. This type of behaviour is observed only in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




