
What is meant by an ideal gas and a real gas? What are the causes of the deviation from ideal gas behavior? Plot a graph between pressure and volume for ideal gases and real gases.
Answer
569.7k+ views
Hint:If we know whether the gas is ideal or real, we can predict the behavior of their intermolecular attractive forces, and the volume occupied by those gases within a system. An Ideal gas, as the names go behaves ideally, under any given temperature and pressure.
Complete answer:
What is an ideal gas?
An ideal gas will follow all the gas laws (i.e., Boyle’s law, Charles’s law, and Avogadro’s law), under a very wide range of temperature and pressure. Ideal gas also follows the kinetic theory of gases which states, there will be negligible amount of volume occupied (\[ \approx \]0) by each gas molecule and that there would be no attractive forces between the gas molecules. Both of the statements are incorrect and hence we have the concept of real gases.
What is a real gas?
A real opposite does not follow the gas law under a wide range of temperature and pressure, and it also disobeys the kinetic gas equation. Eg., if we compress a real gas, the pressure will increase between the gas molecules, which will result in the dominance of attractive forces, and the gas will convert into liquid (condensation), which is contradictory to the “no attractive forces” statement by the kinetic theory of gases.
There are two conditions under which a real gas deviates from the ideal gas behavior these are;
1. Low temperature
2. High pressure
The graph of a real and ideal gas is drawn below, at a constant temperature.
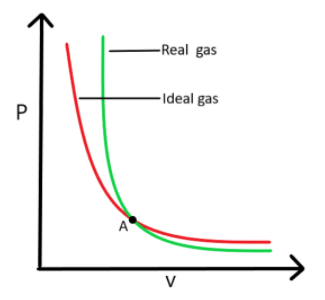
Point ‘A’ in the graph shows the intersection, where the real gas can show ideal behavior.
Note:
The concept of real gas is hypothetical means there is no such gas which can show ideal behaviors under a wide range of temperature and pressure. However, a real gas can show ideal behavior for a shorter range of temperature and pressure, meaning at low pressure and high temperature a real gas can show ideal behavior.
Complete answer:
What is an ideal gas?
An ideal gas will follow all the gas laws (i.e., Boyle’s law, Charles’s law, and Avogadro’s law), under a very wide range of temperature and pressure. Ideal gas also follows the kinetic theory of gases which states, there will be negligible amount of volume occupied (\[ \approx \]0) by each gas molecule and that there would be no attractive forces between the gas molecules. Both of the statements are incorrect and hence we have the concept of real gases.
What is a real gas?
A real opposite does not follow the gas law under a wide range of temperature and pressure, and it also disobeys the kinetic gas equation. Eg., if we compress a real gas, the pressure will increase between the gas molecules, which will result in the dominance of attractive forces, and the gas will convert into liquid (condensation), which is contradictory to the “no attractive forces” statement by the kinetic theory of gases.
There are two conditions under which a real gas deviates from the ideal gas behavior these are;
1. Low temperature
2. High pressure
The graph of a real and ideal gas is drawn below, at a constant temperature.

Point ‘A’ in the graph shows the intersection, where the real gas can show ideal behavior.
Note:
The concept of real gas is hypothetical means there is no such gas which can show ideal behaviors under a wide range of temperature and pressure. However, a real gas can show ideal behavior for a shorter range of temperature and pressure, meaning at low pressure and high temperature a real gas can show ideal behavior.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




