
What is meant by the chelate effect? Give an example.
Answer
593.7k+ views
Hint: Chelate is a term related to coordination compounds. When a di- or polydentate ligand uses its two or more donor atoms together to bind a single metal ion then it is said to be a chelate ligand. The number
of such ligating groups is called denticity of the ligand.
Complete answer: When a ligand is bound to a metal ion through one single donor atom then it is called unidetate but when it uses two donor atoms it is said to be a bidentate ligand. $C{l^ - }$ , ${H_2}O$are unidentate ligands and ${C_2}O_4^ - $ , ${H_2}NC{H_2}C{H_2}N{H_2}$are bidentate ligands. Similarly ligands having more than two donor atoms are called polydentate ligands $N{\left( {C{H_2}C{H_2}N{H_2}} \right)_3}$is a polydentate ligand. When a di- or polydentate ligand uses its two or more donor atoms together to bind a single metal ion then it is said to be a chelate ligand. When a ligand uses its two or more donor atoms simultaneously a ring-like structure is formed. The formation of such ring-like structures is called a chelate effect. Compounds having chelate rings are more stable as compared to other compounds. An example of such a complex is ethylenediamine-cadmium. The structure of this complex is:
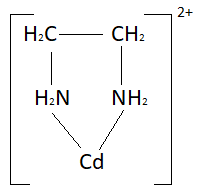
Note:
The coordination number of the metal complex is defined as the number of ligand donor atoms to which metal is directly bonded. The central atom/ion and the ligands attached to it are enclosed in a square bracket and the term collectively used is the coordination sphere.
of such ligating groups is called denticity of the ligand.
Complete answer: When a ligand is bound to a metal ion through one single donor atom then it is called unidetate but when it uses two donor atoms it is said to be a bidentate ligand. $C{l^ - }$ , ${H_2}O$are unidentate ligands and ${C_2}O_4^ - $ , ${H_2}NC{H_2}C{H_2}N{H_2}$are bidentate ligands. Similarly ligands having more than two donor atoms are called polydentate ligands $N{\left( {C{H_2}C{H_2}N{H_2}} \right)_3}$is a polydentate ligand. When a di- or polydentate ligand uses its two or more donor atoms together to bind a single metal ion then it is said to be a chelate ligand. When a ligand uses its two or more donor atoms simultaneously a ring-like structure is formed. The formation of such ring-like structures is called a chelate effect. Compounds having chelate rings are more stable as compared to other compounds. An example of such a complex is ethylenediamine-cadmium. The structure of this complex is:

Note:
The coordination number of the metal complex is defined as the number of ligand donor atoms to which metal is directly bonded. The central atom/ion and the ligands attached to it are enclosed in a square bracket and the term collectively used is the coordination sphere.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




