
Why is the melting point of benzamide more than acetamide?
Answer
563.1k+ views
Hint:In the given question firstly we have to define the structure, shape and the nature of both the amides. This will help us to decide the factors that will make the difference in the melting point of both compounds. Now as we know that the benzamide is much stable and much heavier than the acetamide that makes it much more thermally stable in the direct comparison.
Complete step by step answer:
In the given question we have to first find out the structure and the formula of both the compounds so that we can compare in order to get the answer.
i.Benzamide :
-The formula of the given compound is ${C_7}{H_7}NO$
-The structure of the compound is
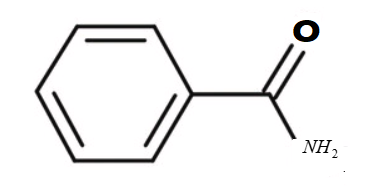
-It is an aromatic compound.
ii. Acetamide :
-The formula of the given compound is $C{H_3}CON{H_2}$
-The structure of the compound is
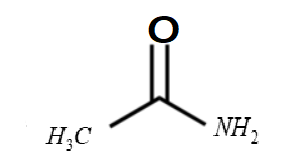
-It is a non aromatic compound.
Now by observing the nature, formula and the structure of the benzamide and acetamide we can state that, both of them contain the same functional group.But we can also observe that the molecular mass of benzamide is much greater than that of acetamide.
Therefore, it will result in benzamide having stronger intermolecular forces. This also emulates that it has higher melting point, due to the direct proportionality.
Note:
Benzamide is a white solid with the properties of an amide. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides exist.
Complete step by step answer:
In the given question we have to first find out the structure and the formula of both the compounds so that we can compare in order to get the answer.
i.Benzamide :
-The formula of the given compound is ${C_7}{H_7}NO$
-The structure of the compound is

-It is an aromatic compound.
ii. Acetamide :
-The formula of the given compound is $C{H_3}CON{H_2}$
-The structure of the compound is
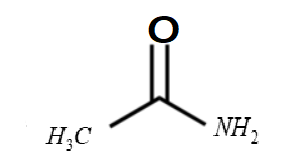
-It is a non aromatic compound.
Now by observing the nature, formula and the structure of the benzamide and acetamide we can state that, both of them contain the same functional group.But we can also observe that the molecular mass of benzamide is much greater than that of acetamide.
Therefore, it will result in benzamide having stronger intermolecular forces. This also emulates that it has higher melting point, due to the direct proportionality.
Note:
Benzamide is a white solid with the properties of an amide. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides exist.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




