
Metals are good conductors of heat and electricity. Why?
Answer
606.3k+ views
Hint: So as to answer this question we should be having a clear idea about the various bonds that are present in a metallic structure. The structure of the metals are very important in order to justify the fact that they are very good conductors of heat and electricity.
Complete step-by-step answer:
The structures of pure metals are simple to describe since the atoms that form these metals can be thought of as identical perfect spheres. More specifically the metallic structure consists of aligned positive ions (cations) in a sea of the delocalized electrons. This means that the electrons are free to move through the structure and gives rise to properties such as conductivity.
Metals contain free moving delocalized electrons when electric voltage is applied and electric fields in the metal trigger the movement of the electrons making them shipped from one end and other end of the conductor electrons will move towards the positive side.
Thus, the metals become a good conductor of electricity.
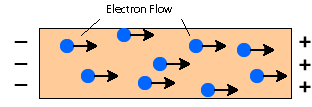
Metal is also a good conductor of heat. Conduction occurs when a substance is heated, particles will get more energy, and vibrate. These molecules then bump into nearby particles and transfer some of their energy to them. This then continues and passes the energy from the hot and down to the cooler end of the substance.
The electrons in metal are delocalized electrons and are free moving electrons so when they gain energy that is heat they vibrate more quickly and can move around, this means that they can pass on the energy more quickly.
Note: Some other properties of metals are, they are malleable (that is they can be beaten into sheets), ductile (can be stretched into wire) and they possess metallic luster.
Complete step-by-step answer:
The structures of pure metals are simple to describe since the atoms that form these metals can be thought of as identical perfect spheres. More specifically the metallic structure consists of aligned positive ions (cations) in a sea of the delocalized electrons. This means that the electrons are free to move through the structure and gives rise to properties such as conductivity.
Metals contain free moving delocalized electrons when electric voltage is applied and electric fields in the metal trigger the movement of the electrons making them shipped from one end and other end of the conductor electrons will move towards the positive side.
Thus, the metals become a good conductor of electricity.

Metal is also a good conductor of heat. Conduction occurs when a substance is heated, particles will get more energy, and vibrate. These molecules then bump into nearby particles and transfer some of their energy to them. This then continues and passes the energy from the hot and down to the cooler end of the substance.
The electrons in metal are delocalized electrons and are free moving electrons so when they gain energy that is heat they vibrate more quickly and can move around, this means that they can pass on the energy more quickly.
Note: Some other properties of metals are, they are malleable (that is they can be beaten into sheets), ductile (can be stretched into wire) and they possess metallic luster.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




