
Methanal and Phenol reacts in the presence of base to give:
A. Bakelite
B. Polyethylene
C. Dacron
D. Nylon-\[6,6\]
Answer
578.4k+ views
Hint: Methanal is also known as formaldehyde where an aldehyde group is attached to \[H\] atom. Phenol is an aromatic alcohol in which a benzene ring is attached to a hydroxy functional group.
Complete step by step answer:
A polymer is a large molecule or macromolecule obtained from small monomers by addition or condensation reactions. The additional polymers are repeating units of hydrocarbons which involve only \[C - C\] bonds between them. The condensation polymers are long chain polymers obtained by condensation of two molecules leaving a molecule of water or alcohol.
The polymer formed by reaction of methanal and phenol is a condensation polymer. The reaction begins by reaction of phenol and formaldehyde in presence of acid or base catalyst. The first step is the formation of ortho and para substituted phenol.
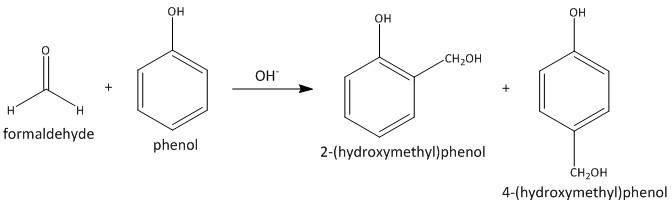
In the second step the ortho isomer reacts with another molecule of the same forming a linear polymer called novolac.
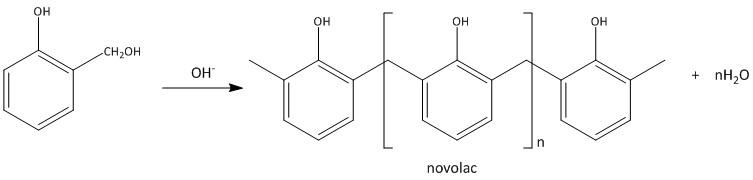
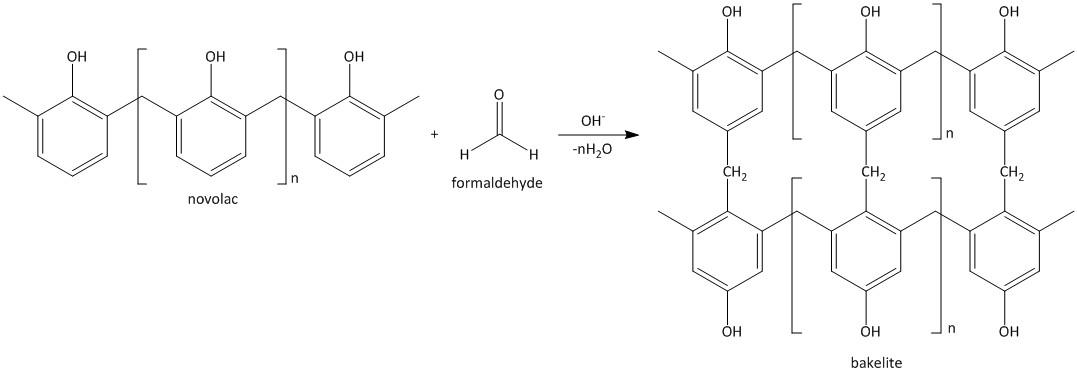
The novolac thus reacts with the formaldehyde to produce cross linked polymer called Bakelite.
Polyethylene: It is an additional polymer of ethene monomer. No molecule is lost during the combination of two ethene molecules. A \[C - C\] bond is formed between the ethene monomer. It is also known as polythene.
Dacron: It is a condensation polymer of ethylene glycol and terephthalic acid. A molecule of water is lost by the combination of the monomers. It is a polyester compound as the linker bond is an ester bond.
Nylon-\[6,6\]: It is a condensation polymer of hexamethylene diamine and adipic acid. A molecule of water is lost by the combination of the monomers. It is a polyamide compound as the linker bond is an amide bond.
Thus option A is the correct answer, i.e. Bakelite.
Note:
The condensation of methanal and phenol is also carried out in presence of acid catalyst. Bakelite is also known as phenol formaldehyde resin, formed by an elimination reaction.
Complete step by step answer:
A polymer is a large molecule or macromolecule obtained from small monomers by addition or condensation reactions. The additional polymers are repeating units of hydrocarbons which involve only \[C - C\] bonds between them. The condensation polymers are long chain polymers obtained by condensation of two molecules leaving a molecule of water or alcohol.
The polymer formed by reaction of methanal and phenol is a condensation polymer. The reaction begins by reaction of phenol and formaldehyde in presence of acid or base catalyst. The first step is the formation of ortho and para substituted phenol.

In the second step the ortho isomer reacts with another molecule of the same forming a linear polymer called novolac.

The novolac thus reacts with the formaldehyde to produce cross linked polymer called Bakelite.

Polyethylene: It is an additional polymer of ethene monomer. No molecule is lost during the combination of two ethene molecules. A \[C - C\] bond is formed between the ethene monomer. It is also known as polythene.
Dacron: It is a condensation polymer of ethylene glycol and terephthalic acid. A molecule of water is lost by the combination of the monomers. It is a polyester compound as the linker bond is an ester bond.
Nylon-\[6,6\]: It is a condensation polymer of hexamethylene diamine and adipic acid. A molecule of water is lost by the combination of the monomers. It is a polyamide compound as the linker bond is an amide bond.
Thus option A is the correct answer, i.e. Bakelite.
Note:
The condensation of methanal and phenol is also carried out in presence of acid catalyst. Bakelite is also known as phenol formaldehyde resin, formed by an elimination reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




