
Methane molecules are nonpolar molecules. Explain.
Answer
560.4k+ views
Hint: The polar molecule is asymmetrical in nature while the non polar is symmetrical in nature. The polar molecule contains electrical poles and non polar does not have electrical poles. In polar molecules the one end has a positive charge while the other end resides a negative charge. While in non polar molecules they do not have profusion of the charges at the opposite ends.
Complete step by step answer:
Let us understand the concept of polarity, and the details of polar and nonpolar molecules to understand this question.
Polarity is a state or condition of an atom which has a positive and a negative charge specifically when it is the case of magnetic or electric poles.
The polarity of the bond of the molecule depends upon the electronegativities of the atoms present for the molecules present in the compound. We have two types of polarity: either a molecule is Polar or a nonpolar molecule.
Now let us understand what are polar and nonpolar molecules.
Polar molecules- a polar molecule is a molecule which is formed when one end of the molecule has more number of positive charges and on the other side the opposite and of the molecule has a negative charge. Due to this difference in charges an electric pole is created.
When a molecule is Polar they have two different centres of positive and negative charges. The centre of negative charge will be found on one side and the centre of positive charge will be found on the other side. In that case the entire molecule will be a polar molecule.
Non polar molecules- A molecule which does not have the charges present at the end because of finely distributed electrons and this distribution which is symmetrical cancel out each other are the non- polar molecules.
The polarity of methane is as shown below:
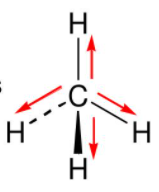
Methane Does not have the charges present at the end because of finely distributed electrons and this distribution which is symmetrical cancel out each other. Hence it is non-polar.
Note: The polarity of the molecule is the state or the condition of the atom or the molecule which have positive and negative charges in electrical poles. It refers to the physical properties of substances such as boiling point. The polarity arises because of the electronegativity difference.
Complete step by step answer:
Let us understand the concept of polarity, and the details of polar and nonpolar molecules to understand this question.
Polarity is a state or condition of an atom which has a positive and a negative charge specifically when it is the case of magnetic or electric poles.
The polarity of the bond of the molecule depends upon the electronegativities of the atoms present for the molecules present in the compound. We have two types of polarity: either a molecule is Polar or a nonpolar molecule.
Now let us understand what are polar and nonpolar molecules.
Polar molecules- a polar molecule is a molecule which is formed when one end of the molecule has more number of positive charges and on the other side the opposite and of the molecule has a negative charge. Due to this difference in charges an electric pole is created.
When a molecule is Polar they have two different centres of positive and negative charges. The centre of negative charge will be found on one side and the centre of positive charge will be found on the other side. In that case the entire molecule will be a polar molecule.
Non polar molecules- A molecule which does not have the charges present at the end because of finely distributed electrons and this distribution which is symmetrical cancel out each other are the non- polar molecules.
The polarity of methane is as shown below:

Methane Does not have the charges present at the end because of finely distributed electrons and this distribution which is symmetrical cancel out each other. Hence it is non-polar.
Note: The polarity of the molecule is the state or the condition of the atom or the molecule which have positive and negative charges in electrical poles. It refers to the physical properties of substances such as boiling point. The polarity arises because of the electronegativity difference.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




