
Methanol on heating with salicylic acid and a few drops of conc. 24HSO gives the smell of:
A. bitter almonds
B. oil of wintergreen
C. rotten egg
D. mustard oil
Answer
570k+ views
Hint: By studying the properties of salicylic acid we came to know that salicylic acid gives an acetylation reaction with methanol in presence of sulphuric acid. The product is an ester of salicylic acid. The product ester has a minty odour.
Complete Step by step answer: Methanol on heating with salicylic acid and a few drops of conc. ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ gives the ester which is methyl $2 - $ hydroxybenzoate. The –COOH is known as the carboxylic group. The RCOOR is known as an ester. The carboxylic group (-COOH) converts into ester $\left( { - {\text{COC}}{{\text{H}}_{\text{3}}}} \right)$ during the reaction of salicylic acid with methanol so, the reaction is known as esterification.
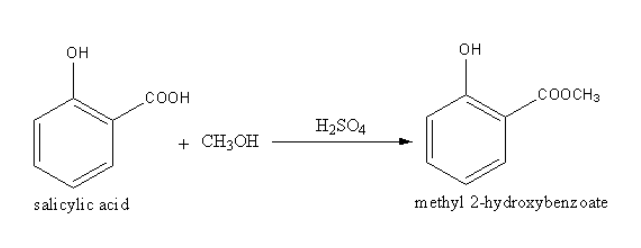
The product methyl $2 - $ hydroxyl benzoate is known as oil of wintergreen. Oil of wintergreen is a naturally produced organic ester.
The structure of oil of wintergreen is as follows:
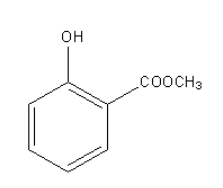
Oil of wintergreen is also known as methyl salicylate because the anion of salicylic acid is known as salicylate which gets attached with the methyl group.
So, methanol on heating with salicylic acid and a few drops of conc. gives the smell of oil of wintergreen.
Many plant species mainly the wintergreen produce the chemical methyl$2 - $ hydroxy benzoate which is known as oil of wintergreen because it is produced from wintergreen plants. It is produced synthetically from salicylic acid and methanol.
Therefore, option (B) oil of wintergreen is correct.
Note: The oil of wintergreen has a minty odour. Oil of wintergreen due to its minty odour use as a flavouring agent, soft drinks, toothpaste, perfumes, etc. it is also used for fragrance. Oil of wintergreen is similar to aspirin. So, just like aspirin, the oil of wintergreen is also used as a pain reliever or anti-inflammatory.
Complete Step by step answer: Methanol on heating with salicylic acid and a few drops of conc. ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ gives the ester which is methyl $2 - $ hydroxybenzoate. The –COOH is known as the carboxylic group. The RCOOR is known as an ester. The carboxylic group (-COOH) converts into ester $\left( { - {\text{COC}}{{\text{H}}_{\text{3}}}} \right)$ during the reaction of salicylic acid with methanol so, the reaction is known as esterification.

The product methyl $2 - $ hydroxyl benzoate is known as oil of wintergreen. Oil of wintergreen is a naturally produced organic ester.
The structure of oil of wintergreen is as follows:

Oil of wintergreen is also known as methyl salicylate because the anion of salicylic acid is known as salicylate which gets attached with the methyl group.
So, methanol on heating with salicylic acid and a few drops of conc. gives the smell of oil of wintergreen.
Many plant species mainly the wintergreen produce the chemical methyl$2 - $ hydroxy benzoate which is known as oil of wintergreen because it is produced from wintergreen plants. It is produced synthetically from salicylic acid and methanol.
Therefore, option (B) oil of wintergreen is correct.
Note: The oil of wintergreen has a minty odour. Oil of wintergreen due to its minty odour use as a flavouring agent, soft drinks, toothpaste, perfumes, etc. it is also used for fragrance. Oil of wintergreen is similar to aspirin. So, just like aspirin, the oil of wintergreen is also used as a pain reliever or anti-inflammatory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




