
Method by which aniline cannot be prepared is:
(A) reduction of nitrobenzene in ${{H}_{2}}/Pd$ in ethanol
(B) potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution
(C) hydrolysis of phenyl isocyanide solution with acidic solution
(D) degradation of benzamide with bromine in alkaline solution
Answer
579.6k+ views
Hint: Draw the structure of aniline. Think about the methods of synthesis of aniline. Then look at the options and write the reactions given and check if aniline is formed as the product in them. The reaction in which aniline is not formed as the product is the answer.
Complete step by step solution:
- Aniline is an aromatic amine. Its molecular formula is ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$ and structure is:
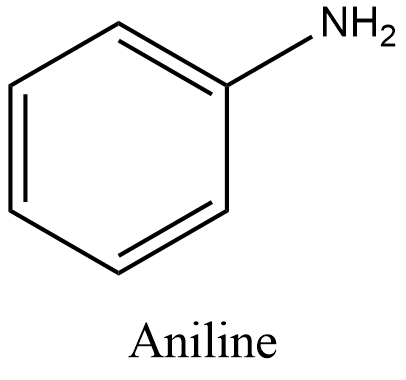
- Now, let’s take a look at the reactions given in the options.
- Reduction of nitrobenzene in ${{H}_{2}}/Pd$ in ethanol gives aniline. The reaction is given as follows,
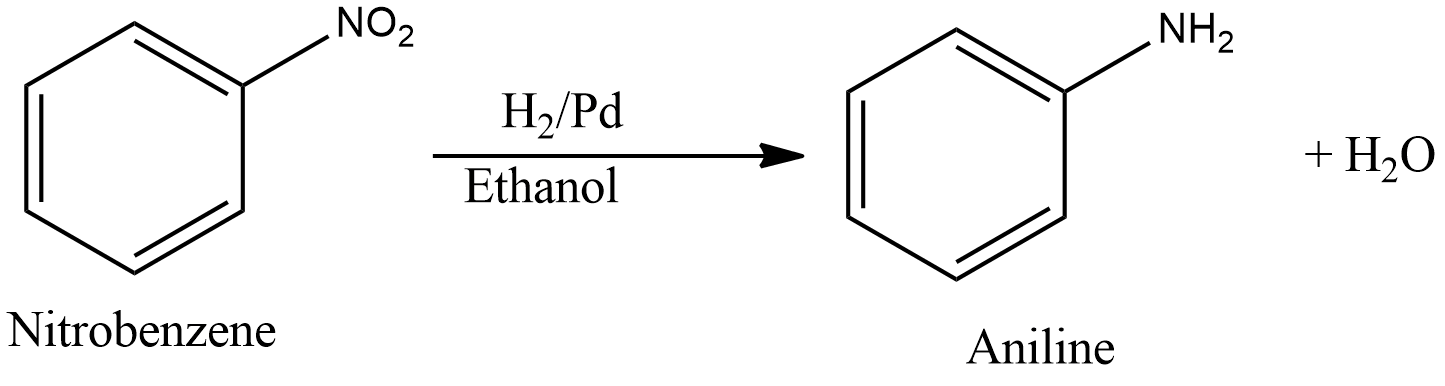
- Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution. This reaction is Gabriel phthalimide synthesis. According to the reaction, only primary amines are formed. Aniline is a primary amine. But one of the drawbacks of this reaction is that aryl halides, that is, chlorobenzene will not undergo nucleophilic substitution reaction with the anion of phthalimide. Therefore, this reaction will not proceed further. General reaction of Gabriel synthesis is given as,
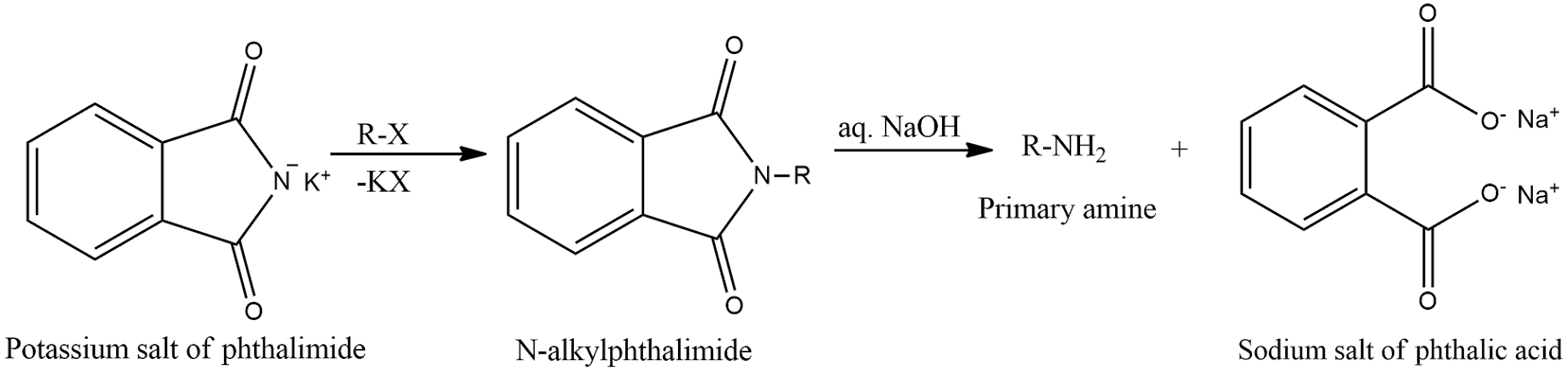
- Hydrolysis of phenyl isocyanide solution with acidic solution gives N-phenyl formamide which on further hydrolysis in acidic conditions gives aniline and formic acid.
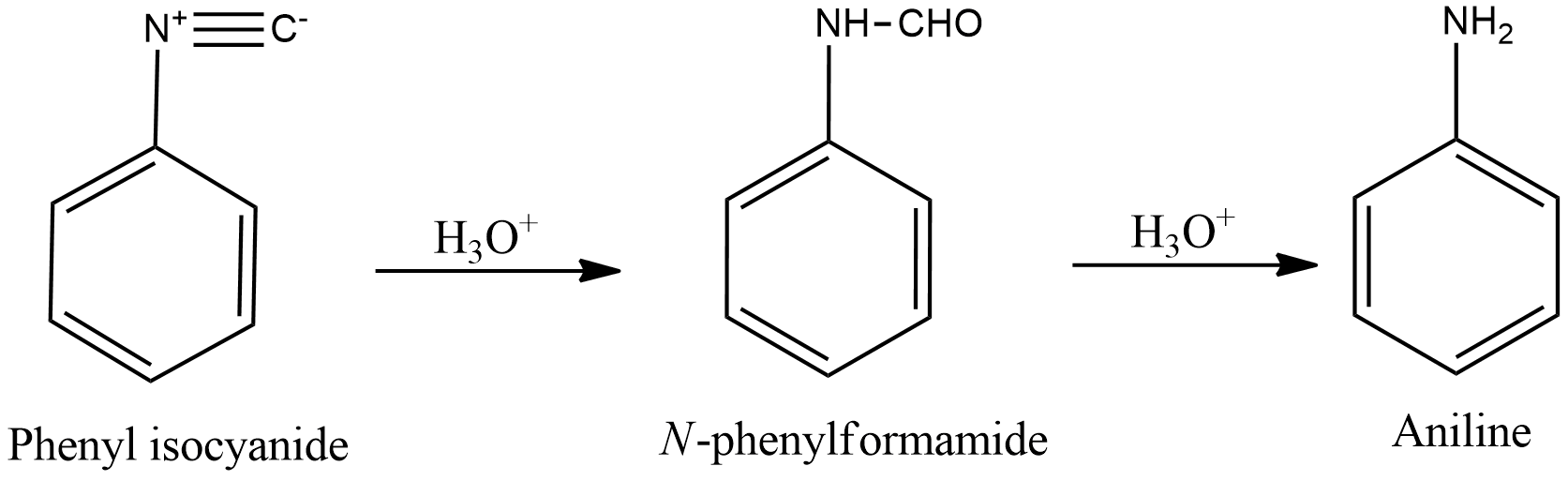
- Degradation of benzamide with bromine in alkaline solution gives aniline. This reaction is Hoffmann bromamide degradation.
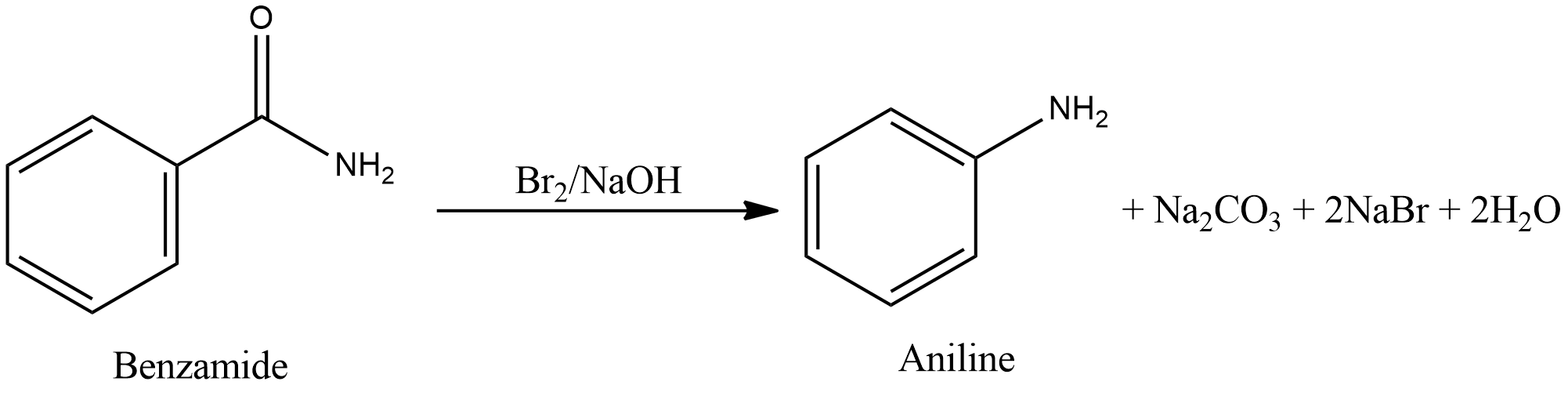
- Therefore, the only reaction in which aniline is not formed is Gabriel phthalimide synthesis.
- Therefore, the answer is option (B).
Note: Remember, in Gabriel phthalimide synthesis, only primary amines are formed as the product. Aromatic amines are not formed in this reaction because aryl halides undergo electrophilic substitution reaction and not nucleophilic substitution reaction which is taking place in this reaction. This reaction is also called N-Alkylation reaction.
Complete step by step solution:
- Aniline is an aromatic amine. Its molecular formula is ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$ and structure is:

- Now, let’s take a look at the reactions given in the options.
- Reduction of nitrobenzene in ${{H}_{2}}/Pd$ in ethanol gives aniline. The reaction is given as follows,

- Potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution. This reaction is Gabriel phthalimide synthesis. According to the reaction, only primary amines are formed. Aniline is a primary amine. But one of the drawbacks of this reaction is that aryl halides, that is, chlorobenzene will not undergo nucleophilic substitution reaction with the anion of phthalimide. Therefore, this reaction will not proceed further. General reaction of Gabriel synthesis is given as,

- Hydrolysis of phenyl isocyanide solution with acidic solution gives N-phenyl formamide which on further hydrolysis in acidic conditions gives aniline and formic acid.

- Degradation of benzamide with bromine in alkaline solution gives aniline. This reaction is Hoffmann bromamide degradation.

- Therefore, the only reaction in which aniline is not formed is Gabriel phthalimide synthesis.
- Therefore, the answer is option (B).
Note: Remember, in Gabriel phthalimide synthesis, only primary amines are formed as the product. Aromatic amines are not formed in this reaction because aryl halides undergo electrophilic substitution reaction and not nucleophilic substitution reaction which is taking place in this reaction. This reaction is also called N-Alkylation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




