
$M{g_2}{C_3}$reacts with water to form propyne ${C_3}{H_4}$ has:
A. Six sigma and two pi bonds
B. Three sigma and one pi bond
C. two sigma and one pi bond
D. two sigma and three pi bonds
Answer
573.9k+ views
Hint: Magnesium carbide reacts with water and produces propyne and magnesium hydroxide. This reaction is also known as hydrolysis of magnesium carbide.We can calculate the number of sigma and pi bonds by drawing the structure of propyne correctly.
\[M{g_2}{C_3}{\rm{ }} + {\rm{ }}4{H_2}O{\rm{ }} \to {\rm{ }}2Mg{\left( {OH} \right)_2}{\rm{ }} + {\rm{ }}{C_3}{H_4}\]
Complete Step by step answer: A covalent bond is formed when the sharing of electrons takes place. The covalent bond is of two types one is sigma bond and other one is pie bond.
Sigma bond-This is a type of covalent bond which is formed by the overlapping of orbitals in an end to end fashion. This type of overlapping is also known as axial overlap. When then it is a s-s overlapping and it is also a sigma bond. Similarly, when two p-orbitals overlap then it is a s-p overlapping and it is also an example of sigma bond.
Pi-bond-This is another type of covalent bond which is formed by the overlapping of orbitals in a way that it is parallel to each other and perpendicular to the internuclear axis. This type of overlapping is also known as lateral overlap. When there is a formation of two p orbital overlap. When then it is a p-p overlapping and it is also a pi-bond.
The structure of propyne is
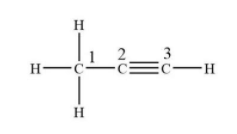
We can clearly see that carbon-1 forms 3 sigma bonds with hydrogen and one sigma bond with carbon numbers. And carbon-2 forms two sigma and two pi-bond while carbon-3 forms only two sigma and two pi-bonds.
In total there are six sigma and two pi bonds.
Hence option (A) Six sigma and two pi bonds, is the correct option.
Note: The Propyne is an alkyne with three carbon atoms. From the structural formula of propyne it is clear that there is one triple and five single bonds. So the number of sigma and pi bonds can be determined with the help of structure itself. Also, due to the greater extent of overlapping in case of sigma bond the sigma bond is stronger than pi bond as it involves lateral overlapping.
\[M{g_2}{C_3}{\rm{ }} + {\rm{ }}4{H_2}O{\rm{ }} \to {\rm{ }}2Mg{\left( {OH} \right)_2}{\rm{ }} + {\rm{ }}{C_3}{H_4}\]
Complete Step by step answer: A covalent bond is formed when the sharing of electrons takes place. The covalent bond is of two types one is sigma bond and other one is pie bond.
Sigma bond-This is a type of covalent bond which is formed by the overlapping of orbitals in an end to end fashion. This type of overlapping is also known as axial overlap. When then it is a s-s overlapping and it is also a sigma bond. Similarly, when two p-orbitals overlap then it is a s-p overlapping and it is also an example of sigma bond.
Pi-bond-This is another type of covalent bond which is formed by the overlapping of orbitals in a way that it is parallel to each other and perpendicular to the internuclear axis. This type of overlapping is also known as lateral overlap. When there is a formation of two p orbital overlap. When then it is a p-p overlapping and it is also a pi-bond.
The structure of propyne is

We can clearly see that carbon-1 forms 3 sigma bonds with hydrogen and one sigma bond with carbon numbers. And carbon-2 forms two sigma and two pi-bond while carbon-3 forms only two sigma and two pi-bonds.
In total there are six sigma and two pi bonds.
Hence option (A) Six sigma and two pi bonds, is the correct option.
Note: The Propyne is an alkyne with three carbon atoms. From the structural formula of propyne it is clear that there is one triple and five single bonds. So the number of sigma and pi bonds can be determined with the help of structure itself. Also, due to the greater extent of overlapping in case of sigma bond the sigma bond is stronger than pi bond as it involves lateral overlapping.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




