
How might one draw an atomic and molecular orbital diagram?
Answer
526.8k+ views
Hint: Sub-atomic orbital graphs are outlines of sub-atomic orbital (MO) energy levels, appeared as short flat lines in the middle, flanked by constituent nuclear orbital (AO) energy levels for correlation, with the energy levels expanding from the base to the top.
Complete step by step solution:
The Molecular Orbital Theory, at first created by Robert S. Mullikan, consolidates the wave-like attributes of electrons in portraying holding conduct. In Molecular Orbital Theory, the holding between iotas is portrayed as a blend of their nuclear orbitals.
While the Valence Bond Theory and Lewis Structures adequately clarify straightforward models, the Molecular Orbital Theory gives answers to more perplexing inquiries.
In the Molecular Orbital Theory, the electrons are delocalized. Electrons are viewed as delocalized when they are not appointed to a specific particle or bond (as for the situation with Lewis Structures). All things considered, the electrons are "spread out" across the atom.
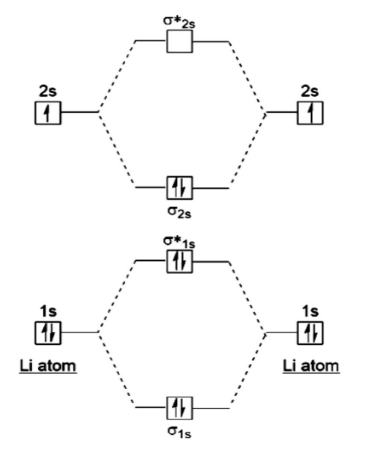
The Molecular Orbital Theory permits one to foresee the conveyance of electrons in an atom which thus can help anticipate subatomic properties like shape, attraction, and Bond Order.
Molecules structure bonds by sharing electrons. Molecules can share two, four, or six electrons, shaping single, twofold, and triple bonds individually. Despite the fact that it is difficult to decide the specific situation of an electron, it is conceivable to compute the likelihood that one will discover the electron anytime around the core utilizing the Schrödinger Equation.
Note:
Drawing atomic orbital graphs is one of the trickier ideas in science. The principal significant advance is understanding the distinction between two significant hypotheses: Valence Bond Theory and Molecular Orbital Theory.
Complete step by step solution:
The Molecular Orbital Theory, at first created by Robert S. Mullikan, consolidates the wave-like attributes of electrons in portraying holding conduct. In Molecular Orbital Theory, the holding between iotas is portrayed as a blend of their nuclear orbitals.
While the Valence Bond Theory and Lewis Structures adequately clarify straightforward models, the Molecular Orbital Theory gives answers to more perplexing inquiries.
In the Molecular Orbital Theory, the electrons are delocalized. Electrons are viewed as delocalized when they are not appointed to a specific particle or bond (as for the situation with Lewis Structures). All things considered, the electrons are "spread out" across the atom.

The Molecular Orbital Theory permits one to foresee the conveyance of electrons in an atom which thus can help anticipate subatomic properties like shape, attraction, and Bond Order.
Molecules structure bonds by sharing electrons. Molecules can share two, four, or six electrons, shaping single, twofold, and triple bonds individually. Despite the fact that it is difficult to decide the specific situation of an electron, it is conceivable to compute the likelihood that one will discover the electron anytime around the core utilizing the Schrödinger Equation.
Note:
Drawing atomic orbital graphs is one of the trickier ideas in science. The principal significant advance is understanding the distinction between two significant hypotheses: Valence Bond Theory and Molecular Orbital Theory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




