
What is the molecular formula of a diamond?
Answer
528.3k+ views
Hint: Allotropy is the property of any element to exist in more than one form. Diamond, graphite, coke, etc. are called allotropes of carbon. The structure and the position of the atoms in the diamond lattice tell us the molecular formula of diamond and the nature of its bonds.
Complete answer:
Allotropes are the different forms that are possessed by an element that has different structural variations and chemical variations. The most common allotropes can be seen in carbon. Carbon possesses allotropy and has different forms like, graphite, diamond, coke, graphenes, fullerenes, etc. These forms consist of carbon as a sole atom which has different arrangement patterns in their respective crystal lattice.
Diamond is one of the allotropes of carbon that is a crystalline, solid, and homogenous form of carbon. Diamond consists of a 3 – dimensional structure of carbon network. The carbon atoms are $s{{p}^{3}}$ hybridized and form a tetrahedral geometry. Each carbon atom is linked with four other carbon atoms which are again linked with four carbons, which forms a network. These carbons are linked together with covalent bonds. The structure of diamond is:
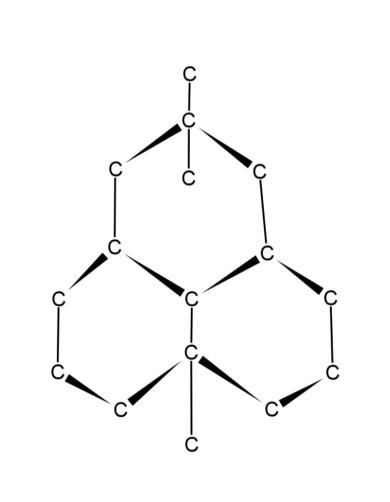
Hence, the molecular formula of diamond is C as it consists of only carbon atoms.
Note:
In diamond carbon is linked with 4 other carbons, so it has a C – 4 arrangement, while in graphite, the carbon atoms are linked with 6 other carbons, so it has a C – 6 arrangement. These are the crystalline allotropes of carbon. While coke, coal and soot are termed as amorphous allotropes of carbon.
Complete answer:
Allotropes are the different forms that are possessed by an element that has different structural variations and chemical variations. The most common allotropes can be seen in carbon. Carbon possesses allotropy and has different forms like, graphite, diamond, coke, graphenes, fullerenes, etc. These forms consist of carbon as a sole atom which has different arrangement patterns in their respective crystal lattice.
Diamond is one of the allotropes of carbon that is a crystalline, solid, and homogenous form of carbon. Diamond consists of a 3 – dimensional structure of carbon network. The carbon atoms are $s{{p}^{3}}$ hybridized and form a tetrahedral geometry. Each carbon atom is linked with four other carbon atoms which are again linked with four carbons, which forms a network. These carbons are linked together with covalent bonds. The structure of diamond is:

Hence, the molecular formula of diamond is C as it consists of only carbon atoms.
Note:
In diamond carbon is linked with 4 other carbons, so it has a C – 4 arrangement, while in graphite, the carbon atoms are linked with 6 other carbons, so it has a C – 6 arrangement. These are the crystalline allotropes of carbon. While coke, coal and soot are termed as amorphous allotropes of carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




