
Molecular mass of water is ______ amu.
Answer
517.2k+ views
Hint: The word "relative molecular mass" is more formally established. As described, relative atomic and molecular mass values are dimensionless. In fact, however, the adjective 'relative' is absent since atomic and molecular masses are universally considered to be relative to the mass of $^{12}C$.
Complete answer:
The atomic masses of each nuclide present in the molecule are used to measure molecular masses, while the regular atomic weights of each element are used to calculate molar masses. The isotopic composition of the element in a given sample is taken into account by the regular atomic weight (usually assumed to be "normal").
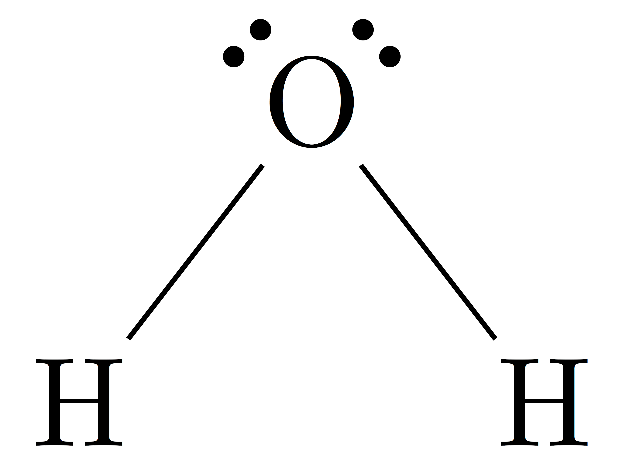
Water molecule structure
Molecular formula = \[{H_2}O\]
Atomic mass of Hydrogen = 1 amu
Atomic mass of 2 Hydrogen = 2 amu
Atomic mass of Oxygen = 16 amu
Molecular mass of Water = 16 + 2 = 18 amu
A mass equal to one twelfth the mass of a carbon-12 atom is known as an atomic mass unit. The mass of any element's isotope is measured in comparison to the carbon-12 standard. Specific atom masses are very thin. It is possible to calculate those minuscule masses using a modern instrument known as a mass spectrometer.
Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent. It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its chemical formula is \[{H_2}O\], meaning that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. Two hydrogen atoms are attached to one oxygen atom at an angle of \[104.45^\circ .\]
Note:
The liquid state of \[{H_2}O\] at normal temperature and pressure is referred to as "water." Precipitation in the form of rain and aerosols in the form of fog are also generated by it. Clouds are made up of floating water droplets and ice in its stable state. Crystalline ice can precipitate in the form of snow when finely separated. Steam or water vapour is the gaseous state of water.
Complete answer:
The atomic masses of each nuclide present in the molecule are used to measure molecular masses, while the regular atomic weights of each element are used to calculate molar masses. The isotopic composition of the element in a given sample is taken into account by the regular atomic weight (usually assumed to be "normal").

Water molecule structure
Molecular formula = \[{H_2}O\]
Atomic mass of Hydrogen = 1 amu
Atomic mass of 2 Hydrogen = 2 amu
Atomic mass of Oxygen = 16 amu
Molecular mass of Water = 16 + 2 = 18 amu
A mass equal to one twelfth the mass of a carbon-12 atom is known as an atomic mass unit. The mass of any element's isotope is measured in comparison to the carbon-12 standard. Specific atom masses are very thin. It is possible to calculate those minuscule masses using a modern instrument known as a mass spectrometer.
Water is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent. It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its chemical formula is \[{H_2}O\], meaning that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. Two hydrogen atoms are attached to one oxygen atom at an angle of \[104.45^\circ .\]
Note:
The liquid state of \[{H_2}O\] at normal temperature and pressure is referred to as "water." Precipitation in the form of rain and aerosols in the form of fog are also generated by it. Clouds are made up of floating water droplets and ice in its stable state. Crystalline ice can precipitate in the form of snow when finely separated. Steam or water vapour is the gaseous state of water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




