
Molisch reagent is used to identify the following compound?
A. Glucose
B. Raffinose
C. D-deoxyribose
D. All of these
Answer
608.4k+ views
Hint: Molisch reagent is a reagent which is used in the identification of carbohydrates. The reaction is positive only if the carbohydrate reacts with an acid to give a reddish-purple coloured complex.
Complete step by step answer:
Molisch's test is a chemical test, used to detect the presence of carbohydrates. This test is based on the dehydration of the carbohydrate by an acid (we generally use sulphuric acid or hydrochloric acid) to produce an aldehyde, which condenses with two molecules of a phenol usually α-naphthol (other phenols such as resorcinol and thymol also give coloured products), which gives a reddish-purple coloured ring.
The procedure for Molisch’s test is –
Add 2-3 drops of Molisch’s reagent to a small amount of the analyte in a test tube.
Mix it well.
Now, add a few drops of concentrated sulfuric acid dropwise along the walls of the test tube(to facilitate the formation of a layer and to avoid mixing).
The development of a purple ring at the layer formed by the concentrated acid is a positive indication for the test.
If no purple or reddish-purple colour is formed, the given analyte does not contain any carbohydrate, and the test is therefore negative.
Amongst the given options, glucose gives a positive result for Molisch’s test. The reaction is as given below –
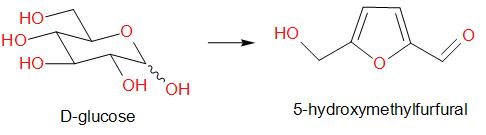
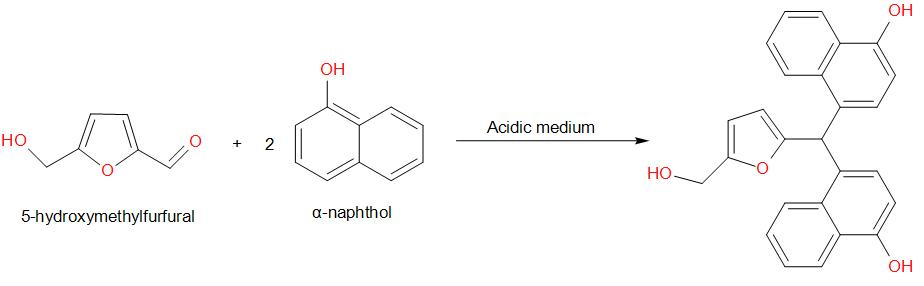
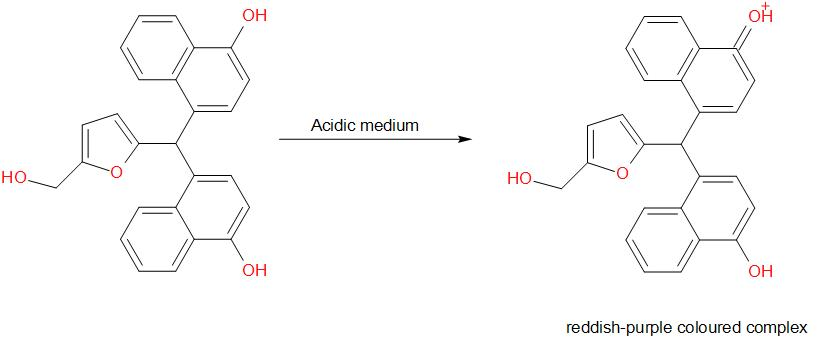
Therefore, the answer is option (a) – Glucose.
Additional information: Molisch’s test is named after Austrian botanist Hans Molisch.
Note: The result for Molisch’s test is positive reaction for almost all carbohydrates (exceptions include tetroses & trioses). Also, some glycoproteins and nucleic acids because they undergo hydrolysis when exposed to strong mineral acids. They react to form monosaccharides. And therefore, show a positive result for Molisch’s test.
Complete step by step answer:
Molisch's test is a chemical test, used to detect the presence of carbohydrates. This test is based on the dehydration of the carbohydrate by an acid (we generally use sulphuric acid or hydrochloric acid) to produce an aldehyde, which condenses with two molecules of a phenol usually α-naphthol (other phenols such as resorcinol and thymol also give coloured products), which gives a reddish-purple coloured ring.
The procedure for Molisch’s test is –
Add 2-3 drops of Molisch’s reagent to a small amount of the analyte in a test tube.
Mix it well.
Now, add a few drops of concentrated sulfuric acid dropwise along the walls of the test tube(to facilitate the formation of a layer and to avoid mixing).
The development of a purple ring at the layer formed by the concentrated acid is a positive indication for the test.
If no purple or reddish-purple colour is formed, the given analyte does not contain any carbohydrate, and the test is therefore negative.
Amongst the given options, glucose gives a positive result for Molisch’s test. The reaction is as given below –



Therefore, the answer is option (a) – Glucose.
Additional information: Molisch’s test is named after Austrian botanist Hans Molisch.
Note: The result for Molisch’s test is positive reaction for almost all carbohydrates (exceptions include tetroses & trioses). Also, some glycoproteins and nucleic acids because they undergo hydrolysis when exposed to strong mineral acids. They react to form monosaccharides. And therefore, show a positive result for Molisch’s test.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




