
What is the monomer unit of natural rubber?
(a)- Isoprene
(b)- Ethane
(c)- Neoprene
(d)- Tetrafluoroethene
Answer
233.1k+ views
Hint: The natural rubber is chemically called Polyisoprene. The cis-polyisoprene is called natural rubber and trans-isoprene is called Gutta-percha.
Complete step by step answer:
There are 2 two types of rubber: natural rubber and synthetic rubber.
The natural rubber has remarkable elasticity and undergoes long-range reversible extension even under a relatively small applied force. That is why it is also called an elastomer. It is manufactured from latex which is a colloidal solution of rubber particles in water. Latex is obtained by making an incision in the bark of rubber trees found in tropical and subtropical countries such as Southern India (Kerala, Tamil Nadu, Karnataka, etc), Malaysia, Sri Lanka, etc.
Chemically, natural rubber is a linear 1,4-addition polymer of isoprene (i.e., 2-methyl-1,3-butadiene).
The structure of isoprene is:
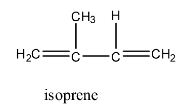
And the polymerization reaction of isoprene to form Polyisoprene is given below:
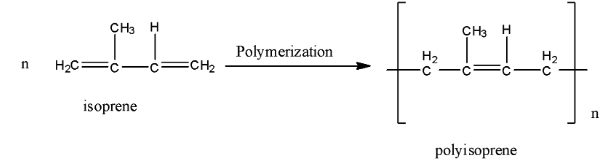
Since each repeating unit in polyisoprene contains a double bond, it may have either a cis- or a trans- orientation. Actually, in natural rubber, all the double bonds have cis- stereochemistry. In other words, natural rubber is cis-polyisoprene. In contrast, synthetic rubber (gutta percha) is trans-polyisoprene. The structure of natural rubber and gutta-percha is given below:
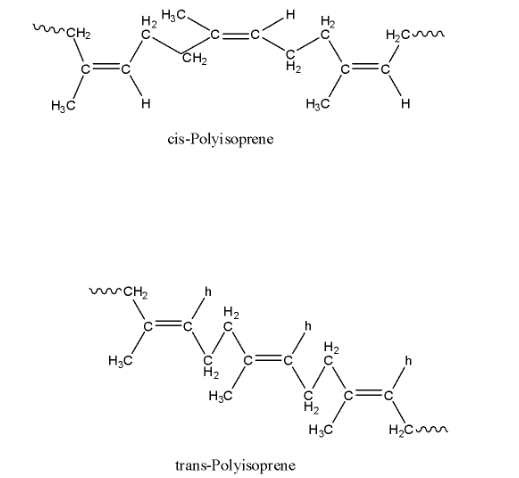
Inspection of the structure of natural rubber reveals that there are no polar groups/substituents and hence intermolecular forces of attraction are only weak van der Waals interaction. These forces are further weakened because of the cis-configuration of all the double bonds which does not allow the polymer chains to come close enough for effective interactions. Thus, cis-polyisoprene does not have a straight-chain but has a coiled structure. As a result, it can be stretched like a spring. Thus, the natural rubber is elastic.
Hence, the correct answer is an option (a)- Isoprene.
Note: Due to the coiled structure, natural rubber does not fit closely in the crystal lattice and hence is considered to be non-crystalline. In contrast, due to highly zig-zag structure, gutta-percha fits closely in the crystal lattice and hence, is considered as crystalline.
Complete step by step answer:
There are 2 two types of rubber: natural rubber and synthetic rubber.
The natural rubber has remarkable elasticity and undergoes long-range reversible extension even under a relatively small applied force. That is why it is also called an elastomer. It is manufactured from latex which is a colloidal solution of rubber particles in water. Latex is obtained by making an incision in the bark of rubber trees found in tropical and subtropical countries such as Southern India (Kerala, Tamil Nadu, Karnataka, etc), Malaysia, Sri Lanka, etc.
Chemically, natural rubber is a linear 1,4-addition polymer of isoprene (i.e., 2-methyl-1,3-butadiene).
The structure of isoprene is:

And the polymerization reaction of isoprene to form Polyisoprene is given below:

Since each repeating unit in polyisoprene contains a double bond, it may have either a cis- or a trans- orientation. Actually, in natural rubber, all the double bonds have cis- stereochemistry. In other words, natural rubber is cis-polyisoprene. In contrast, synthetic rubber (gutta percha) is trans-polyisoprene. The structure of natural rubber and gutta-percha is given below:

Inspection of the structure of natural rubber reveals that there are no polar groups/substituents and hence intermolecular forces of attraction are only weak van der Waals interaction. These forces are further weakened because of the cis-configuration of all the double bonds which does not allow the polymer chains to come close enough for effective interactions. Thus, cis-polyisoprene does not have a straight-chain but has a coiled structure. As a result, it can be stretched like a spring. Thus, the natural rubber is elastic.
Hence, the correct answer is an option (a)- Isoprene.
Note: Due to the coiled structure, natural rubber does not fit closely in the crystal lattice and hence is considered to be non-crystalline. In contrast, due to highly zig-zag structure, gutta-percha fits closely in the crystal lattice and hence, is considered as crystalline.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




