
What is the monomer unit of Teflon?
(A)
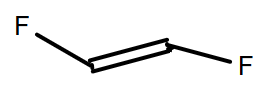
(B)
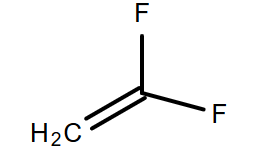
(C)
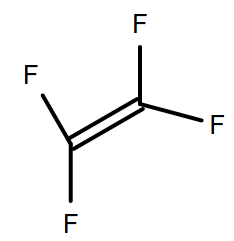
(D)
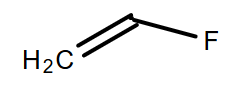




Answer
584.4k+ views
Hint: Monomers as the name tells that they are the single unit which polymerises to form a long chain of the monomer, known as polymers. Teflon is also a polymer, whose monomers have a minimum of 4 fluorine atoms.
Complete step by step solution:
-In the given question, we have to identify the structure of the monomer of the Teflon.
-Now, as we know that Teflon is a polymer which is made when many small units repeat and make a bond with each other to form a long chain.
-The structure of Teflon includes a long chain of carbon which has fluorines in place of the hydrogen atoms.
-Due to the replacement of all the hydrogen atoms by fluorine they have some different properties such as they become resistant from the attack of any reagent.
-So, the monomer of Teflon will be tetrafluoroethylene. Because four fluorine atoms are present so named as 'tetrafluoro' and the carbon are bonded with each other with a double bond so named as 'ethene'.
-The chemical reaction showing the polymerisation of tetrafluoroethylene to form teflon is
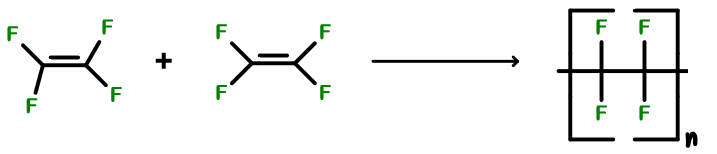
-Here, we can see the structure of teflon which is made up of so many molecules of tetrafluoroethylene by the polymerisation reaction.
-That’s why option C will be the correct answer.
-Moreover, as we know that there is the absence of a hydrogen atom in the monomer of the Teflon so, in option A, B and D are incorrect because they all consist of hydrogen atoms.
Therefore, option (C) is the correct answer.
Note: The structure of tetrafluoro ethene is much similar to the ethylene because they both are responsible for forming the long chain of ethene except the presence of fluorine in Teflon. Overheating of Teflon can be harmful to humans.
Complete step by step solution:
-In the given question, we have to identify the structure of the monomer of the Teflon.
-Now, as we know that Teflon is a polymer which is made when many small units repeat and make a bond with each other to form a long chain.
-The structure of Teflon includes a long chain of carbon which has fluorines in place of the hydrogen atoms.
-Due to the replacement of all the hydrogen atoms by fluorine they have some different properties such as they become resistant from the attack of any reagent.
-So, the monomer of Teflon will be tetrafluoroethylene. Because four fluorine atoms are present so named as 'tetrafluoro' and the carbon are bonded with each other with a double bond so named as 'ethene'.
-The chemical reaction showing the polymerisation of tetrafluoroethylene to form teflon is

-Here, we can see the structure of teflon which is made up of so many molecules of tetrafluoroethylene by the polymerisation reaction.
-That’s why option C will be the correct answer.
-Moreover, as we know that there is the absence of a hydrogen atom in the monomer of the Teflon so, in option A, B and D are incorrect because they all consist of hydrogen atoms.
Therefore, option (C) is the correct answer.
Note: The structure of tetrafluoro ethene is much similar to the ethylene because they both are responsible for forming the long chain of ethene except the presence of fluorine in Teflon. Overheating of Teflon can be harmful to humans.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




