
Most stable sigma complex among the following is:
A.
B.
C.
D.




Answer
510k+ views
Hint: We need to know that the chemical stability is otherwise known as thermodynamic stability. And the stability of the compound depends on some factors like lattice energy, solvation energy, sublimation energy etc. And the stability also depends on the resonance structure of a compound and the size of the compound. If an electron withdrawing group is present in a compound, the compound becomes more stable.
Complete answer:
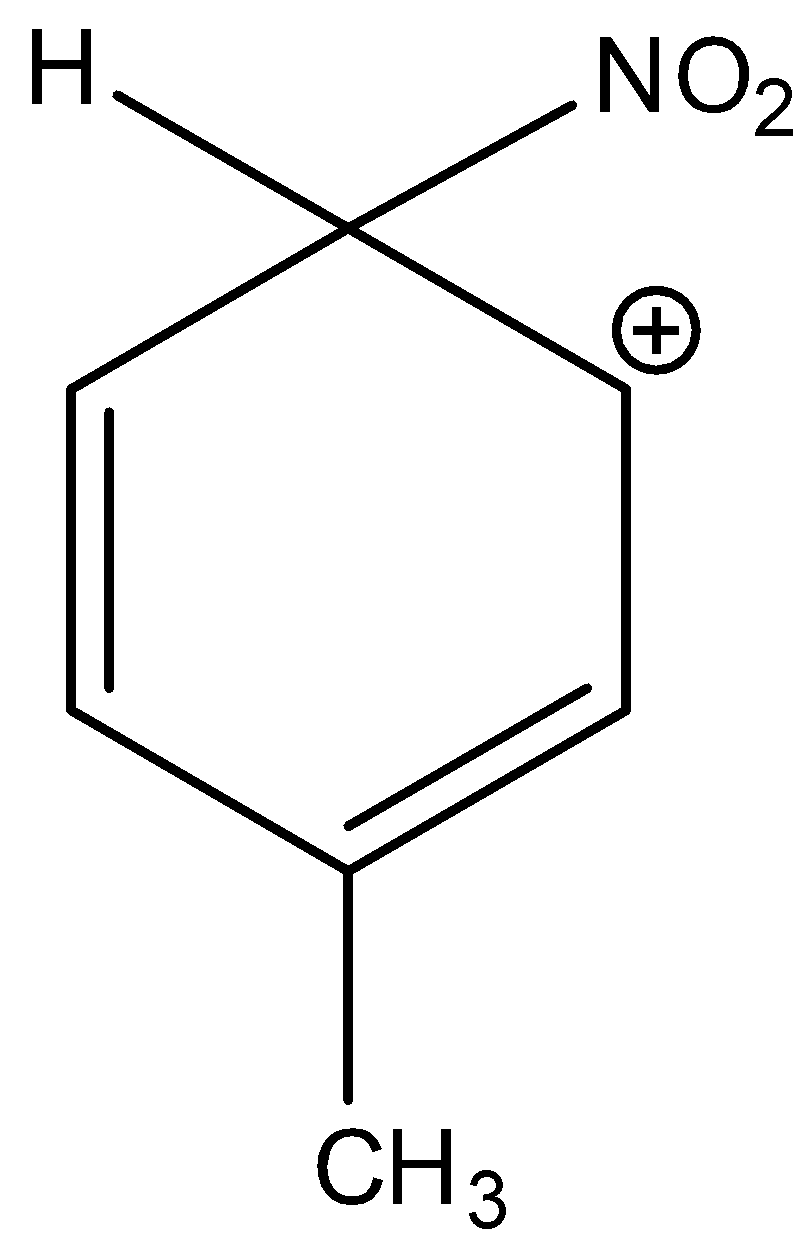
Here the complex is 3-methyl-6-nitrocyclohexa-2,4-dien-1-ylium. The methyl group is attached to the benzene ring. And it is not the most stable sigma complex. Hence, option (A) is incorrect.
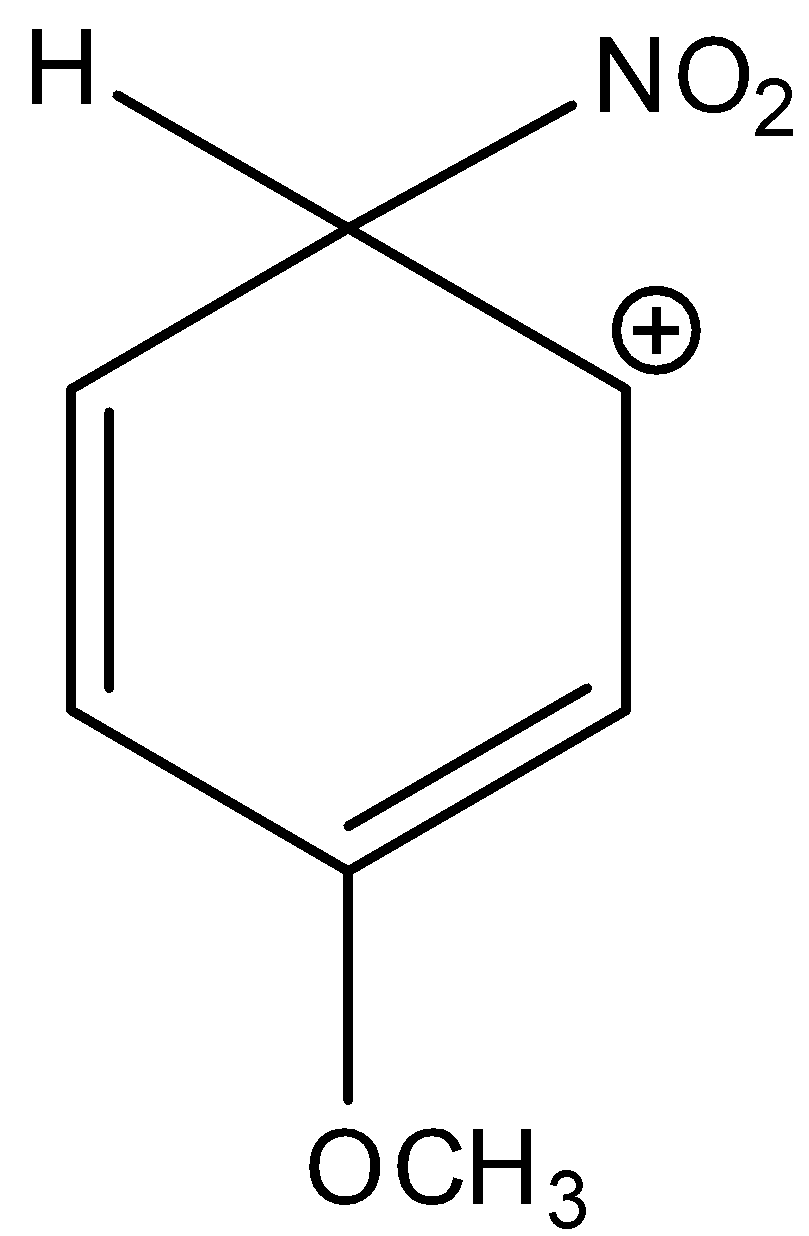
Here, the given compound is 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium. The methoxy group is attached in the third position of the benzene ring and the nitro group is also attached in this complex. And this methoxy group is ortho or para directing and an electron withdrawing group. There are two lone pairs present in the oxygen of methoxy group. Thus, it is more electronegative. And, it has a +M effect due to resonance. So, 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium is a more stable sigma complex. Let’s see the resonance,
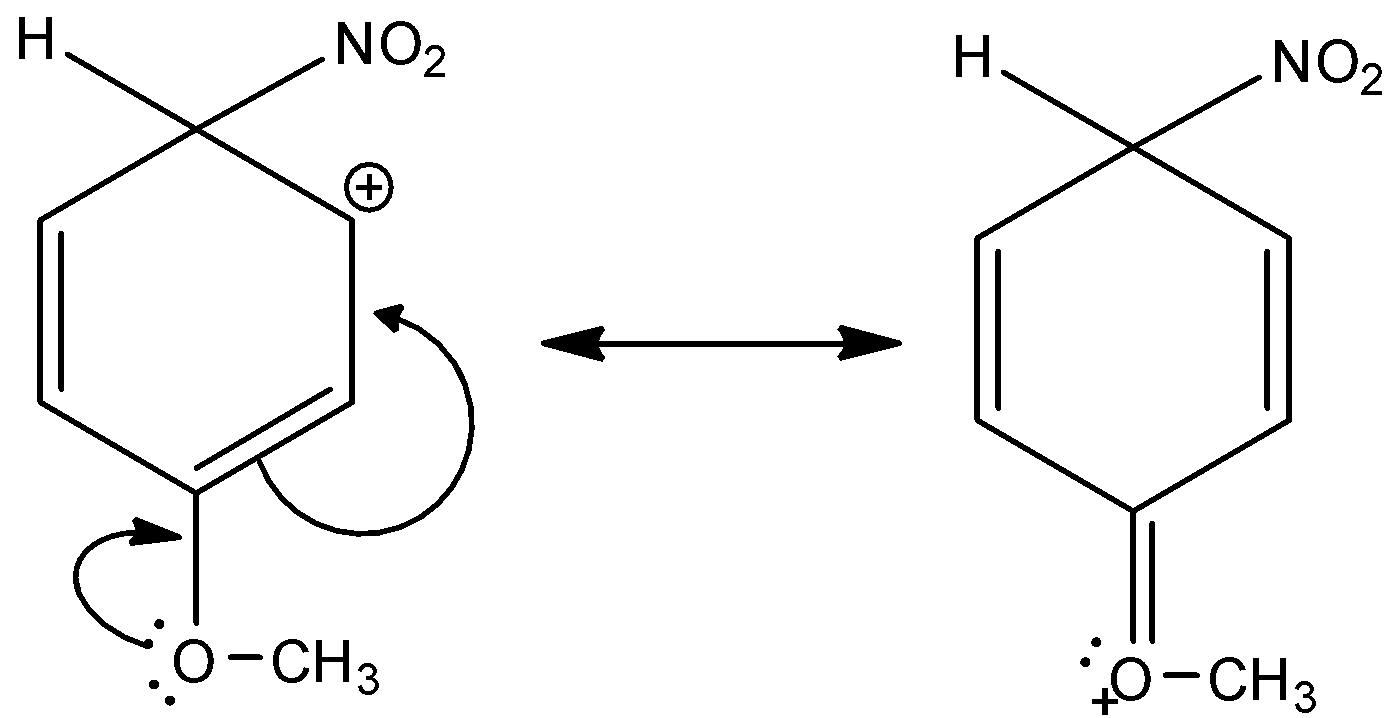
Hence, option (B) is correct.
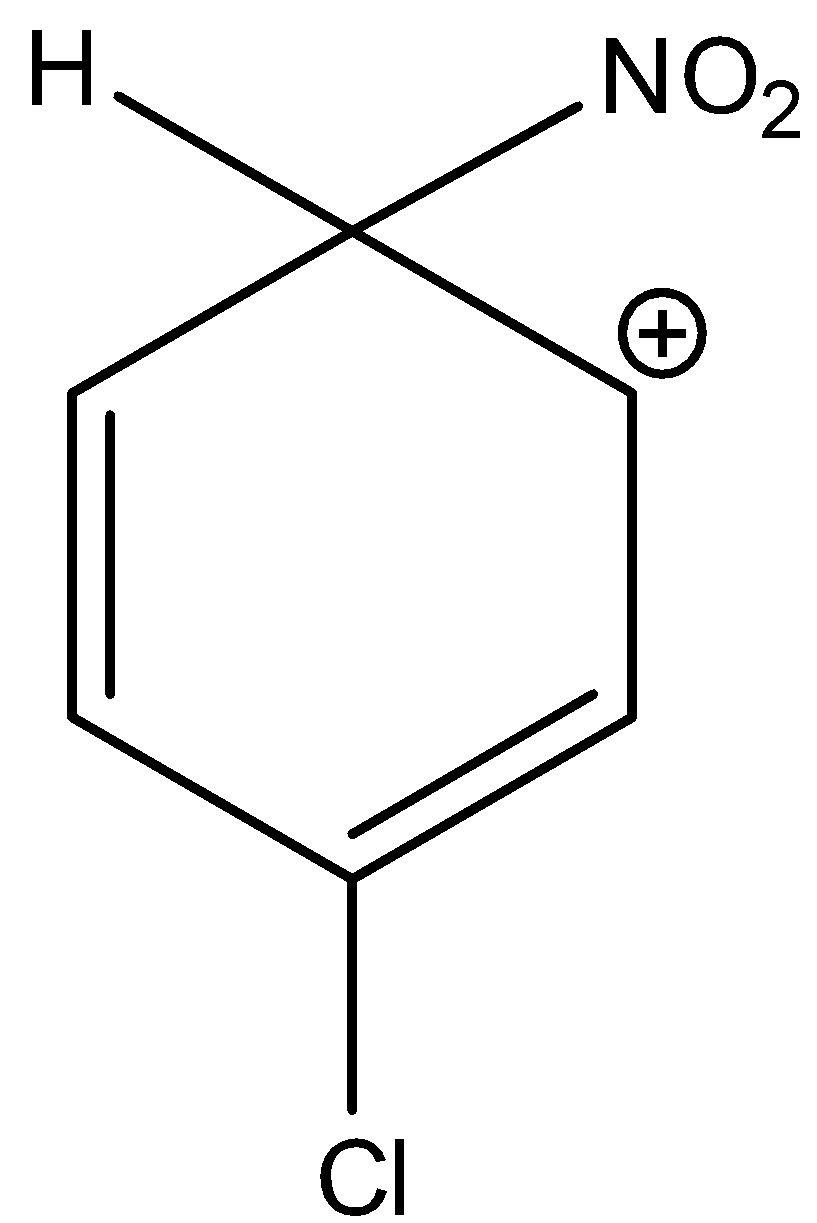
3-chloro-6-nitrocyclohexa-2,4-dien-1-ylium is not a stable sigma complex. Hence, option (C) is incorrect.
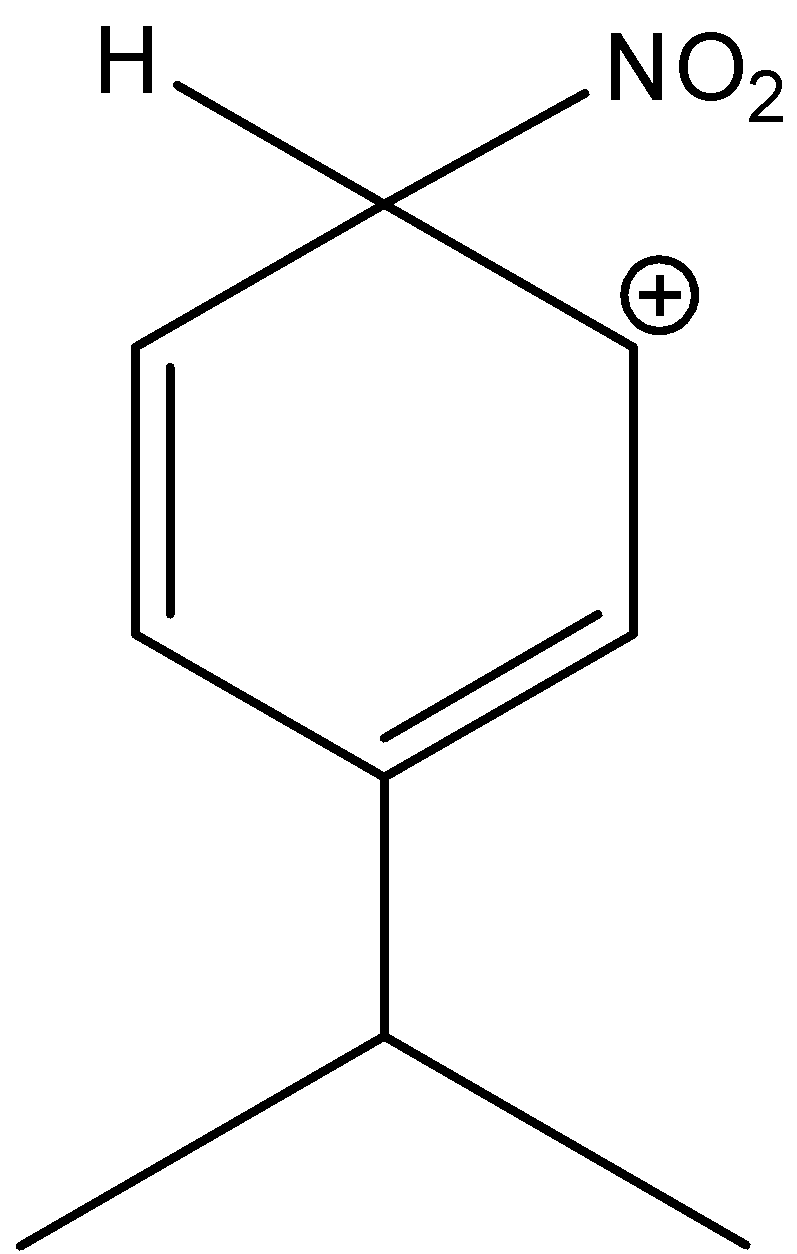
3-isopropyl-6-nitrocyclohexa-2,4-dien-1-ylium is not the stable structure of the sigma complex. Hence, the option (D) is incorrect.
Hence, option (B) is correct.
Note:
If the nitration occurs at the ortho or para position, the sigma complex becomes more stable. The nitronium ion is reacting with the benzene and there is a formation of a sigma complex by the loss of a proton or hydrogen atom and an aromatic product. If there is a formation of a new sigma bond in the initial stage, that intermediate is known as the sigma complex.
Complete answer:
Here the complex is 3-methyl-6-nitrocyclohexa-2,4-dien-1-ylium. The methyl group is attached to the benzene ring. And it is not the most stable sigma complex. Hence, option (A) is incorrect.
Here, the given compound is 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium. The methoxy group is attached in the third position of the benzene ring and the nitro group is also attached in this complex. And this methoxy group is ortho or para directing and an electron withdrawing group. There are two lone pairs present in the oxygen of methoxy group. Thus, it is more electronegative. And, it has a +M effect due to resonance. So, 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium is a more stable sigma complex. Let’s see the resonance,

Hence, option (B) is correct.
3-chloro-6-nitrocyclohexa-2,4-dien-1-ylium is not a stable sigma complex. Hence, option (C) is incorrect.
3-isopropyl-6-nitrocyclohexa-2,4-dien-1-ylium is not the stable structure of the sigma complex. Hence, the option (D) is incorrect.
Hence, option (B) is correct.
Note:
If the nitration occurs at the ortho or para position, the sigma complex becomes more stable. The nitronium ion is reacting with the benzene and there is a formation of a sigma complex by the loss of a proton or hydrogen atom and an aromatic product. If there is a formation of a new sigma bond in the initial stage, that intermediate is known as the sigma complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




