
“\[{N_3}^{ - }\;\] is stable due to resonance.”
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Answer
583.8k+ views
Hint:
The question demands that we should know the structure of \[{N_3}^{ - }\;\] and the meaning of the resonance to decide if the statements are true or false.
Complete step by step solution
Resonance is seen where there is availability of the two electrons (said to be lone pairs of electrons), which will resonate from one atom to another.
The resonance structures show that the electrons are delocalized within the molecule and through this process, the molecule gains extra stability. Azides, \[{N_3}^{ - }\;\] are energy-rich molecules because the extra electron on nitrogen atoms creates the negative charge on either terminal of central nitrogen which can be delocalized by resonance. As we are aware that nitrogen has the ability to form four bonds due to its half-filled p-orbitals, so the azide shows resonance as electrons can jump from one orbit of nitrogen to another.
\[{N_3}^{ - }\,\] can exist in a stable state due to resonance the same as benzene is stable due to resonance of p-orbital electrons. The resonance structure is shown below.
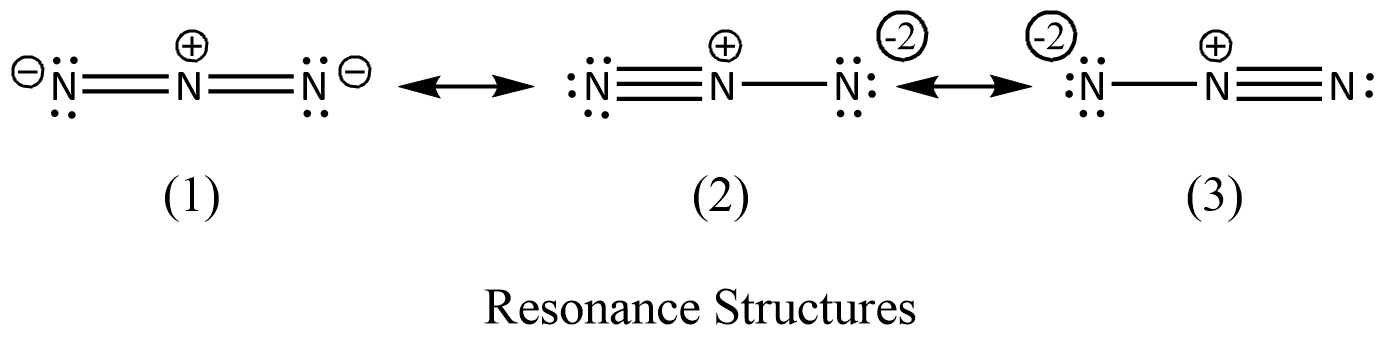
As you can see the structure, if we count the number of total electrons, it is $22$. It is isoelectronic with \[{N{O_2}^{ + }}\]. It is also known as pseudo halogen.
The structure is as shown in the figure.
Hence, the correct answer is 1.
Note:
The similar structure can be seen for ozone and for organic structure benzene. Sodium Azide is used in air bags which get exploded when required, in the air bags the nitrogen is produced from the explosion of the sodium azide triggered by electric charge.
The question demands that we should know the structure of \[{N_3}^{ - }\;\] and the meaning of the resonance to decide if the statements are true or false.
Complete step by step solution
Resonance is seen where there is availability of the two electrons (said to be lone pairs of electrons), which will resonate from one atom to another.
The resonance structures show that the electrons are delocalized within the molecule and through this process, the molecule gains extra stability. Azides, \[{N_3}^{ - }\;\] are energy-rich molecules because the extra electron on nitrogen atoms creates the negative charge on either terminal of central nitrogen which can be delocalized by resonance. As we are aware that nitrogen has the ability to form four bonds due to its half-filled p-orbitals, so the azide shows resonance as electrons can jump from one orbit of nitrogen to another.
\[{N_3}^{ - }\,\] can exist in a stable state due to resonance the same as benzene is stable due to resonance of p-orbital electrons. The resonance structure is shown below.
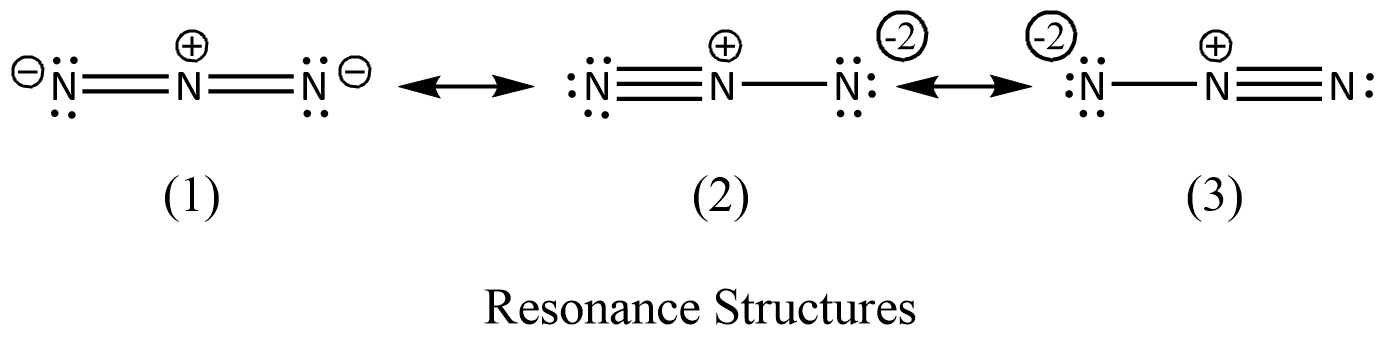
As you can see the structure, if we count the number of total electrons, it is $22$. It is isoelectronic with \[{N{O_2}^{ + }}\]. It is also known as pseudo halogen.
The structure is as shown in the figure.
Hence, the correct answer is 1.
Note:
The similar structure can be seen for ozone and for organic structure benzene. Sodium Azide is used in air bags which get exploded when required, in the air bags the nitrogen is produced from the explosion of the sodium azide triggered by electric charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




