
Why is the NaCl structure more stable than the CsCl structure?
Answer
497.4k+ views
Hint: Approach this question with the concept of electronegativity and the difference in the electronegativities of both the structures. As we know, if there is a high electronegativity difference between two atoms of an ionic compound, they tend to be very unstable whereas if the electronegativity difference is low, then the ionic compound has a stable form.
Complete answer:
Let us discuss about electronegativity and its concept followed by telling the reason behind NaCl structure being more stable than the CsCl structure as follows:
Electronegativity: It is the tendency of an atom to attract shared pairs of electrons or electron density (cloud) towards itself. The electronegativity of an atom is affected by both its atomic number as well as the distance at which its valence electrons reside from the nucleus.
-For the periodic table, the trend of electronegativity increases from left to right in the period and decreases from top to bottom in the group.
-Also if the electronegativity difference between the elements of a compound is more, then they are more unstable but if the electronegativity difference between the elements of the compound is less, then they are considered to be a more stable compound.
-As we know that both NaCl and CsCl have the same chloride ion but different cations. So we will compare them to tell the trend as follows:
Sodium (Na) being at the top of the group is comparatively more electronegative (less electropositive) as compared to cesium (Ce) which lies at the bottom of the alkali group.
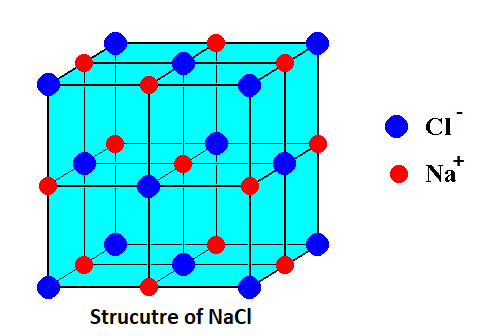
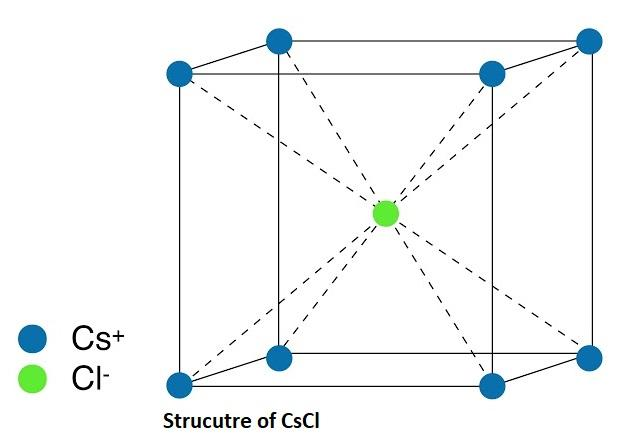
- So, the electronegativity difference between Na and Cl is less as compared to the difference between Cs and Cl which means sodium chloride is more stable than cesium chloride.
-Hence, we can say that the NaCl structure is more stable than the CsCl structure because the electronegativity difference is less in NaCl compound compared to the later one.
Note:
-Remember to learn and understand the concept of all kinds of periodic trends applied on the periodic table along with the elements of it. Also, there are various exceptions in these trends which are necessary to be learned so as to easily deal with these types of questions.
Complete answer:
Let us discuss about electronegativity and its concept followed by telling the reason behind NaCl structure being more stable than the CsCl structure as follows:
Electronegativity: It is the tendency of an atom to attract shared pairs of electrons or electron density (cloud) towards itself. The electronegativity of an atom is affected by both its atomic number as well as the distance at which its valence electrons reside from the nucleus.
-For the periodic table, the trend of electronegativity increases from left to right in the period and decreases from top to bottom in the group.
-Also if the electronegativity difference between the elements of a compound is more, then they are more unstable but if the electronegativity difference between the elements of the compound is less, then they are considered to be a more stable compound.
-As we know that both NaCl and CsCl have the same chloride ion but different cations. So we will compare them to tell the trend as follows:
Sodium (Na) being at the top of the group is comparatively more electronegative (less electropositive) as compared to cesium (Ce) which lies at the bottom of the alkali group.


- So, the electronegativity difference between Na and Cl is less as compared to the difference between Cs and Cl which means sodium chloride is more stable than cesium chloride.
-Hence, we can say that the NaCl structure is more stable than the CsCl structure because the electronegativity difference is less in NaCl compound compared to the later one.
Note:
-Remember to learn and understand the concept of all kinds of periodic trends applied on the periodic table along with the elements of it. Also, there are various exceptions in these trends which are necessary to be learned so as to easily deal with these types of questions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




