
How do you name cycloalkanes with double bonds?
Answer
540.3k+ views
Hint :Cycloalkanes are basically cyclic hydrocarbons means that the carbon of the cycloalkane is arranged in a ring form. If there is a double bond present in the cyclic hydrocarbons then they are called cycloalkenes. Cycloalkenes are a class of organic compounds and we know that for nomenclature or naming of any organic compounds we need to follow the rules specified by the IUPAC.
Complete Step By Step Answer:
Let us see how we can do the nomenclature of the cycloalkenes which contain the halogen in them as a substituent or functional group.
Rule 1: Determine the parent chain. This is the basic step for nomenclature of all cycloalkenes irrespective of the substituent attached to them. A parent chain is the one which has the highest number of carbon atoms.
If there are two cycloalkenes in a compound then always consider the chain with greater number of carbon atoms.
Rule 2: If there is a straight chain attached with the cycloalkenes and that chain has a greater number of carbon atoms as compared to the cycloalkene form, then consider the straight chain for the numbering. Always remember that cycloalkene which acts as substituent for the alkyl straight chain has an ending with ‘’-yl’’ and, hence it is named as cycloalkyl.
Rule 3: Next step is to determine any substituents/functional group (here halogen) attached to the compound.
Rule 4: Now, Start numbering the carbon chain, in such a way that the carbon with functional group (here halogen) or alkyl substituents gets the lowest number. Always remember that a carbon with multiple substituents should have a lower number as compared to the carbon with only one functional group or substituents.
In the above example we did numbering in such a way that substituents got the lowest number possible. And while numbering always make sure that the priority functional group gets the lowest number.
Rule 6: When you name the cycloalkenes then the substituents should be named in alphabetical order.
Rule 7: While writing the name the parent chain name has to be modified when the compound has a double bond. We modify it by substituting the -ane in cycloalkane to -ene in cycloalkene. Always remember that suffix ‘’ene’’ is used in the case of double bonds.
While writing the name firstly write the position of the functional group or substituents and then mention their name, and at last write the parent carbon chain name. but always follow the alphabetical order.
Rule 8: Always remember that a dash should be placed between the name and the number of the substituents.
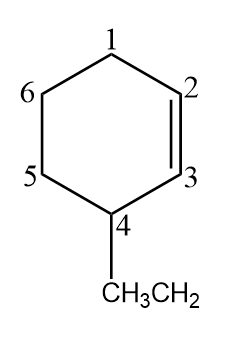
Now let us consider the above cycloalkene. We did the numbering within the chain as the chain itself contains the maximum of the six carbons in it. While numbering we remembered that alkene is the functional group and ethyl is the substituent and thus, they both got the lowest possible number.
we can see that the double bond is at carbon 2. An ethyl group is at carbon 4. So, we name the compound $\text{4-ethyl-cyclo-2-hexene }$or this can also be written as $\text{4-ethyl-cyclo-hex-2-ene }$. Both are acceptable.
Note :
Always remember that whenever a compound contains more than one cycloalkene ring then always consider the ring with maximum number of carbon atoms for numbering. While numbering, see every possible chain or ring which contains the maximum number of atoms and then choose wisely.
Complete Step By Step Answer:
Let us see how we can do the nomenclature of the cycloalkenes which contain the halogen in them as a substituent or functional group.
Rule 1: Determine the parent chain. This is the basic step for nomenclature of all cycloalkenes irrespective of the substituent attached to them. A parent chain is the one which has the highest number of carbon atoms.
If there are two cycloalkenes in a compound then always consider the chain with greater number of carbon atoms.
Rule 2: If there is a straight chain attached with the cycloalkenes and that chain has a greater number of carbon atoms as compared to the cycloalkene form, then consider the straight chain for the numbering. Always remember that cycloalkene which acts as substituent for the alkyl straight chain has an ending with ‘’-yl’’ and, hence it is named as cycloalkyl.
Rule 3: Next step is to determine any substituents/functional group (here halogen) attached to the compound.
Rule 4: Now, Start numbering the carbon chain, in such a way that the carbon with functional group (here halogen) or alkyl substituents gets the lowest number. Always remember that a carbon with multiple substituents should have a lower number as compared to the carbon with only one functional group or substituents.

In the above example we did numbering in such a way that substituents got the lowest number possible. And while numbering always make sure that the priority functional group gets the lowest number.
Rule 6: When you name the cycloalkenes then the substituents should be named in alphabetical order.
Rule 7: While writing the name the parent chain name has to be modified when the compound has a double bond. We modify it by substituting the -ane in cycloalkane to -ene in cycloalkene. Always remember that suffix ‘’ene’’ is used in the case of double bonds.
While writing the name firstly write the position of the functional group or substituents and then mention their name, and at last write the parent carbon chain name. but always follow the alphabetical order.
Rule 8: Always remember that a dash should be placed between the name and the number of the substituents.

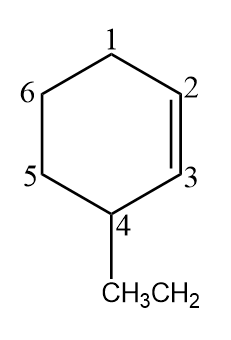
Now let us consider the above cycloalkene. We did the numbering within the chain as the chain itself contains the maximum of the six carbons in it. While numbering we remembered that alkene is the functional group and ethyl is the substituent and thus, they both got the lowest possible number.
we can see that the double bond is at carbon 2. An ethyl group is at carbon 4. So, we name the compound $\text{4-ethyl-cyclo-2-hexene }$or this can also be written as $\text{4-ethyl-cyclo-hex-2-ene }$. Both are acceptable.
Note :
Always remember that whenever a compound contains more than one cycloalkene ring then always consider the ring with maximum number of carbon atoms for numbering. While numbering, see every possible chain or ring which contains the maximum number of atoms and then choose wisely.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




